Spencer Fox Eccles School of Medicine
86 Pre-Surgery Inflammatory and Angiogenesis Biomarkers as Predictors of 12-Month Cancer-Related Distress: Results from the Colocare Study
Clara Lindley; Jennifer Ose; Biljana Gigic; Anita R Peoples; Claire J Han; Tengda Lin; Caroline Himbert; Christy A Warby; Jeurgen Boehm; Sheetal Hardikar; Anjelica Ashworth; Martin Schneider; Alexis Ulrich; Petra Schrotz-King; Jane C Figueiredo; Christopher Li; David Shibata; Erin M Siegel; Adetunji T Toriola; Cornelia M Ulrich; and Karen L Syrjala
Faculty Mentor: Jennifer Ose (Population Health Sciences, University of Utah)
Abstract
Background: Patients with colorectal cancer commonly suffer from complex psychological distress. Elevated distress may be linked to systemic biomarkers. We investigated associations of biomarkers of inflammation andangiogenesis with cancer-related distress (CTXD) score.
Methods: N = 315 patients (stage I-IV) from 2 centers of the ColoCare Study were included: Huntsman Can cerInstitute and University of Heidelberg. Biomarkers (e.g., IL6, VEGF- A, VEGF-D) were measured in serum collected pre-surgery and 12 months thereafter. The CTXD overall score and 4 subscales were collected 12 months after surgery and dichotomized toinvestigate biomarkers as predictors of distress 12 months after surgery; adjusted for age, sex, body mass index, tumor stage, center, and baseline levels of biomarkers.
Results: Doubling of IL6 predicted future increased risk of overall distress [odds ratio (OR), 1.20; 95% confidence interval (CI), 1.02-1.41; P = 0.03]. VEGF-A-predicted future increased risk of high family strain (VEGF-A: OR, 1.21; 95% CI, 1.01-1.44; P = 0.04) and VEGF- D was associated with medical and financial demands(OR, 1.34; 95% CI, 1.01-1.74; P = 0.03).
Conclusions: This is the first study to show that systemic biomarkers are significantly associated with future CTXD score. Distress was not measured at baseline; we cannot rule out ongoing associations of inflammation and distress throughout treatment versus a direct effect of inflammation on distress. Nonetheless, these data add to evidence that biobehavioral processes interact and that systemic biomarkers are associated with cancer-related distress one year after surgery.
Impact: Exercise and diet interventions that lower systemic cytokine levels may impact longer- term CTXD score and improve quality of life of patients with colorectal cancer.
Introduction
Colorectal cancer (CRC) is the third most diagnosed cancer in both men and women in the UnitedStates and is estimated to account for 149,500 new diagnoses and 52,580 deaths (1,2). While improvement of cancer screenings and treatments have resulted in increased survival rates, CRC patients are likely to suffer from distress including depression and anxiety as a result of the disease process and cancer treatment
(3). The National Comprehensive Cancer Network (NCNN) defined cancer-related distress as a “multi-factorial unpleasant emotional experience of psychological (cognitive, behavioral, emotional), social,and/or spiritual nature that may interfere with the ability to cope effectively with cancer, its physicalsymptoms and its treatment” (4). When cancer-related distress is unrecognized or undertreated, patients may experience impaired decision making, decreased satisfaction, depression, anxiety, isolation, panic, and existential and spiritual crisis (5).
Underlying mechanisms of cancer-related distress remain unclear, but an understanding of these mechanisms is essential to develop targeted interventions to reduce distress in this vulnerable population. Systemic inflammation, a hallmark of cancer, is one hypothesized mechanism as it has been associated with psychological distress, including depression (6-8). Consistent evidence indicates that antidepressant drugs possess anti-inflammatory properties (9-12). A randomized clinical trial with n=241 patients withmajor depressive disorder, concluded that lower C-reactive protein (CRP) concentration at enrollment predicted greater improvement of depressive symptoms after 12 weeks (p<0.05), suggesting that inflammation plays a role in the progression of depression (12).
Cancer-related distress can have a complex relationship with symptoms that may influence clinical outcomes. We have previously shown that younger age, advanced cancer stage, lower income, lower social support levels, and worse functional status and low quality of life pre-surgery significantly predicted higher cancer-related distress after surgery (13). A pooling of data from 16 prospective cohort studies including n=163,363 CRC patients, demonstrated that greater levels of distress led to higher risk of cancer mortality (14). Another study reported that surgery-induced stress is a powerful factor promoting malignant tumorgrowth involving inflammation, sympathetic nervous system activation and increased cytokine release, including angiogenesis pathways such as vascular endothelial growth factor (VEGF), interleukin-6 (IL-6) and interleukin-8 (IL-8) which increased risk of cancer recurrence (15). In short, there may be important associations between biomarkers of inflammation and angiogenesis with both psychological and physical symptoms in CRC survivors.
Prior studies reported a positive association between elevated proinflammatory biomarkers and behavioral symptoms in the general population. Studies have revealed elevated levels of proinflammatory biomarkers including IL-6, tumor necrosis factor-α (TNF-α), and CRP in patients with increased anxiety (16). A longitudinal study revealed that CRP is a risk marker for development of depression 10 years after follow up in women who had no history of depression at enrollment, independent of factors including adiposity, weight, and body mass index (BMI) (6). Another large-scale prospective study recruited n=10,357 participants with no history of depression and revealed that inflammation was predictive of depression 5 years later (7). Several other longitudinal studies have revealed this directionality in the general population, which may also be present in patients with CRC (8,17).
Although studies have investigated the link between inflammation and distress in cancer populations, research is limited for CRC (18-20). A cross-sectional study correlated stress with higher levels of TNF-α in patients with chronic lymphocytic leukemia (19). Another cross-sectional study showed that different stages of antitumor therapy revealed positive correlations between anxiety and depression with IL-6, IL-8 and TNF-α (18). Research suggests that inflammation, common before and during treatment for cancer patients, interacts with distress, however prospective research is still lacking.
We have previously shown, that in addition to inflammation, elevated angiogenesis biomarkers are associated with poorer clinical outcomes in CRC patients, however limited research has linked angiogenesis biomarkers to cancer-related distress (21,22). In 2006, an in-vivo study in mice reported significantly increased tumor vascularization and enhanced expression of VEGF in experimentally distressed animals using a physical restraint system designed to induce nervous system activity characteristic of chronic stress (23). In 2002, a cross-sectional study revealed that women with ovarian carcinoma who reported higher levels of social support had lower concentrations of VEGF (24). There is evidence that angiogenesis is associated with distress, however, there is a lack in prospective research in CRC patients (22-24).
Based on prior findings of relationships between inflammatory and angiogenesis processes and stress, thisstudy investigates associations between serum concentrations of pre-surgery and 12 months post-surgery inflammatory and angiogenesis-related biomarkers (CRP, serum-amyloid A (SAA), IL-6, IL-8, VEGF-A, VEGF-D, TNF-α, soluble intercellular adhesion molecule-1 (sICAM-1) and soluble vascular cell adhesion molecule-1 (sVCAM-1)) with cancer-related distress 12 months after surgery. We hypothesized that higherpre-surgery inflammation and angiogenesis would be associated with elevated distress at 12-months. We further expected that higher biomarker concentration for inflammation and angiogenesis at the 12-month time point would be associated with elevated cancer-related distress at the 12-month time point.
Methods
Study Population
This study includes patients from two study centers enrolling in the international prospective ColoCare Study (ClinicalTrials.gov Identifier: NCT02328677) (25). The ColoCare Study includes men and women aged 18–89 who were newly diagnosed with stage I-IV colon, rectum, or rectosigmoid cancer priorto surgery and were mentally and physically able to consent and participate in the study (13,25-27). For thepresent study, patients who had previously been recruited, were newly selected given the eligibility criteria. These patients were selected at the National Center for Tumor Diseases (NCT), Heidelberg (HD, Germany), and the Huntsman Cancer Institute (HCI), Salt Lake City (Utah, USA).
For the present study, patients from HCI and HD with measured inflammatory and angiogenesis biomarkers at pre-surgery and at 12-months follow up and who were administered the CTXD score at 12-months follow-up were included in the analyses. All analyses in this manuscript are based on data and biospecimens collected between 2010 and 2019. This study was approved by the ethics committees of the Medical Faculty at the University of Heidelberg and the University of Utah and was conducted in accordance with the 1964 Helsinki Declaration. All subjects provided written informed consent.
Blood Processing and Biomarker Assays
Pre-surgery blood samples were collected from non-fasting patients prior to surgery at HCI and HD.Serum from patients at HCI was extracted from whole blood and stored at −80˚C in 110 µl aliquots until analysis. Serum samples from patients in Heidelberg were extracted within 4 hours of blood draw, stored in 500 µl aliquots at −80˚C and shipped on dry ice to HCI for analysis.
Serum-based assays for multiplexed IL-6, IL-8, TNF-α, CRP, SAA, sICAM-1, sVCAM-1,VEGF-D, and VEGF-A have previously been established on the Mesoscale Discovery Platform (MSD, Rockville, MD, USA) in the Ulrich laboratory at HCI (21). The biomarker panel was selected based onprevious studies which investigated central biomarkers for inflammation and angiogenesis and behavioral outcomes in both the general and cancer population (12,18,22,26-28).
All samples were blinded, run in duplicate, and read on an MSD Sector Imager 2400A. For both sites, VEGF-A and VEGF-D were run on a V-Plex Angiogenesis Panel Human Kit at a dilution of 1:8. Forthe HCI samples, IL-6, IL-8 and TNF-α were run on a V-Plex Proinflammatory Panel 1 Human Kit at a dilution of 1:2 and CRP, SAA, sICAM- 1 and sVCAM-1 were run on a V-Plex Vascular Injury Panel 2 Human Kit at a dilution of 1:2500. For HD, CRP, SAA, sICAM-1 and sVCAM-1 were run on a V-Plex Vascular Injury Panel 2 Human Kit at a dilution of 1:1000. IL-6, IL-8 and TNF-α were run on a custom U-PLEX Proinflammatory Panel at 1:2 and previously reported with lower levels (26,27). The data was subsequently bridged to V-PLEX Proinflammatory Panel 1 levels. Three quality controls (QC) were run on each plate and used to calculate the inter- and intra-plate coefficient of variabilities (CV). The overall inter-plate CV for HD and HCI samples was 9.9% and 9.6% and the intra-plate CV was 4.6% and 3.9%, respectively.
Cancer-Related Distress
Cancer-related distress was evaluated by the Cancer and Treatment Distress (CTXD) score designedto measure worry or distress related to cancer and its treatment (28). The CTXD score has demonstrated sensitivity and specificity to cancer distress, and elevated scores capture nearly all patients with clinical depression and anxiety symptoms in cancer patients (13). The original measure includes 22 items and 5 subscales: uncertainty, family strain, health burden, finances, and medical demands (13,29,30). For this study, to reduce responder burden, we used a brief 13-item version which correlates highly with the 22-item version (r=0.99). The 13-item version included 4 subscales: uncertainty, family strain, health burden, and medical and financial demands, each of which correlates with the longer subscales (r=0.88–0.97). Internal consistency reliability was strong for the overall scale (α=0.92) as well as for subscales (α=0.88 to α=0.78). The CTXD score has demonstrated sensitivity and specificity to cancer distress and is strongly associated with depression, anxiety, and post-traumatic stress symptoms in cancer patients (31-33). Each item (e.g., including the overall scale) was scored on a scale ranging from 0= “none” to 3= “severe” to determine distress or worry in the past week with overall and subscales calculated as mean scores. The overall score and all subscores were dichotomized into high (≥0.90) and low distress (<0.90) based on evidence that this score captures 90% of those with symptoms of clinical depression or anxiety (29,33,34).
Statistical Analysis
Pre-surgery and 12-month biomarker levels, as well as 12-month CTXD scores were included in the primary analysis. Patients who had at least one biomarker measurement and who have completed the CTXD questionnaire were included in the analysis. Biomarker levels were log2-transformed to preventheteroscedasticity. Potential confounding was investigated for age (years), sex (male, female), BMI (kg/m2), tumor stage (I-IV), tumor site (colon, rectal), adjuvant treatment (yes, no), regular non-steroidal anti-inflammatory (NSAID)/aspirin drugs (NSAIDs use at least 1 time per week during the past month (yes, no)), and study center (HCI, HD). The final model was adjusted for covariates of age, sex, BMI, tumor stage, and study center. We further adjusted analysis at the 12-month time point for baseline biomarker levels.
Mean and standard deviation (SD) were calculated for age, BMI, biomarker concentrations andCTXD scores. Frequency and percentages were calculated for sex, tumor stage, neoadjuvant and adjuvanttreatment, surgery (yes, no), tumor site (colon, rectal), and study center.
To investigate associations between pro-inflammatory and angiogenic biomarkers and CTXDscores, Pearson’s partial correlations were conducted. Correlation coefficients were calculated between pre-surgery biomarker concentrations with CTXD scores, and 12 months post-surgery biomarker concentration with CTXD scores. The 12-months analyses were additionally adjusted for baseline biomarker concentrations. To further investigate the effect of the biomarkers, we conducted logistic regressions to evaluate
pre-surgery biomarkers as predictors of elevated cancer-related distress 12 months after surgery. Odds ratios (OR) and 95% confidence intervals (CI) were calculated to determine the associations of doubling of biomarker concentration with risk of high distress. Additional stratified analyses were conducted to determine effect modification by tumor stage (metastatic, non-metastatic) and by study center (HCI, HD).We tested for heterogeneity between study centers to ensure combined analyses including patients from both sites were valid. We performed tertile analyses to confirm whether biomarkers had a linear relationship with the CTXD scores. Statistical analyses were performed using SAS Studio OnDemand for Academics (SAS InstituteInc., Cary, NC, USA), for Google Chrome 27+, version 3.8. All tests were two-sided and considered statistically significant when p<0.05.
Data Availability Statement
The ColoCare Study data are available from colocarestudy_ admin@hci.utah.edu on reasonable request and as described previously on the ColoCare website (https://uofuhealth.utah.edu/hunts man/labs/colocare-consortium/). Our data sharing procedures have been updated and are available online (https://uofuhealth.utah.edu/ huntsman/labs/colocare-consortium/data-sharing/new-projects.php). For any additional questions please contact the ColoCare Study Administrator Team (colocarestudy_admin@hci.utah.edu).
Results
Table 1 summarizes the patient characteristics at baseline (pre-surgery). The mean age of patients was 62 years and mean BMI was 28 kg/m2. Patients were predominantly male (63%). Patientcharacteristics stratified by study center are presented in Table 2. The study centers had similar rates ofadjuvant treatment, comparing HCI and HD (44% versus 45%) and neoadjuvant treatment comparing HCI and HD (27% versus 33%). Patients in HD had lower BMI than patients from HCI (27kg/m2 versus 30 kg/m2; p<0.01) and were older than patients from HCI (62 versus 61; p=0.37).
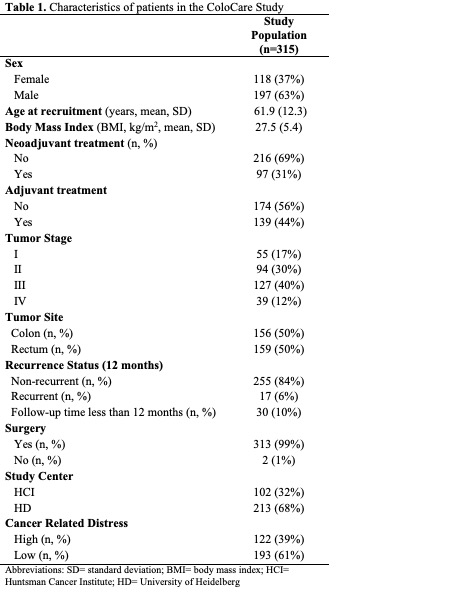
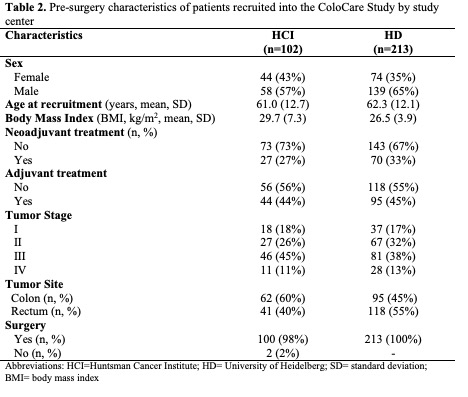
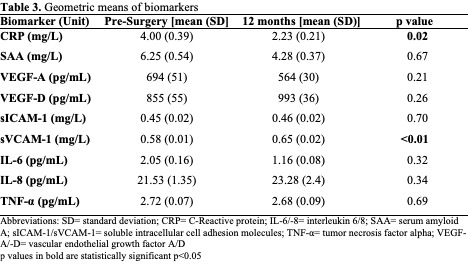
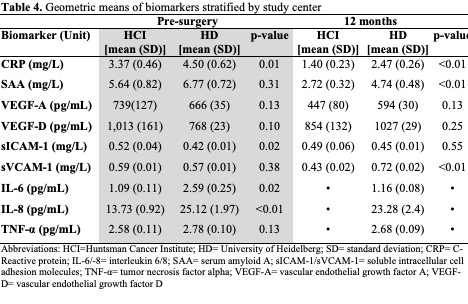
The geometric means of pro-inflammatory and angiogenic biomarkers are presented in Table 3 and separated by study center in Table 4. Pre-surgery, significant differences between centers were observed for CRP, sICAM-1, IL-6, and IL-8 (p<0.01, p=0.02, p=0.02, p<0.01, respectively). 12 months after surgery, significant differences between centers were observed for CRP, SAA, and sVCAM-1 (p<0.01, p<0.01,p<0.01, respectively). [Note, IL-6, IL-8, and TNF-α were not measured in HCI samples at 12- months].
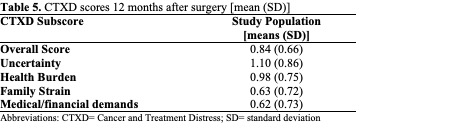
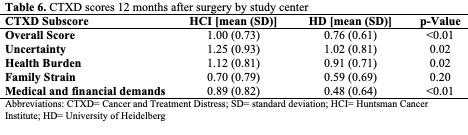
Overall mean CTXD scores are shown in Table 5 and scores stratified by study center are shown in Table 6. Among the 4 subscales, the mean distress for uncertainty was the highest at both study centers. The lowest distress for HCI was family strain, and the lowest distress for HD was managing medical and financial demands. For each subscore and the overall score, patients in the US reported greater distress than in Germany. Significant differences in CTXD-defined distress between sites were observed for the overall score, uncertainty, health burden, and managing medical and financial demands (p<0.01, p=0.02, p=0.02, p<0.01, respectively).
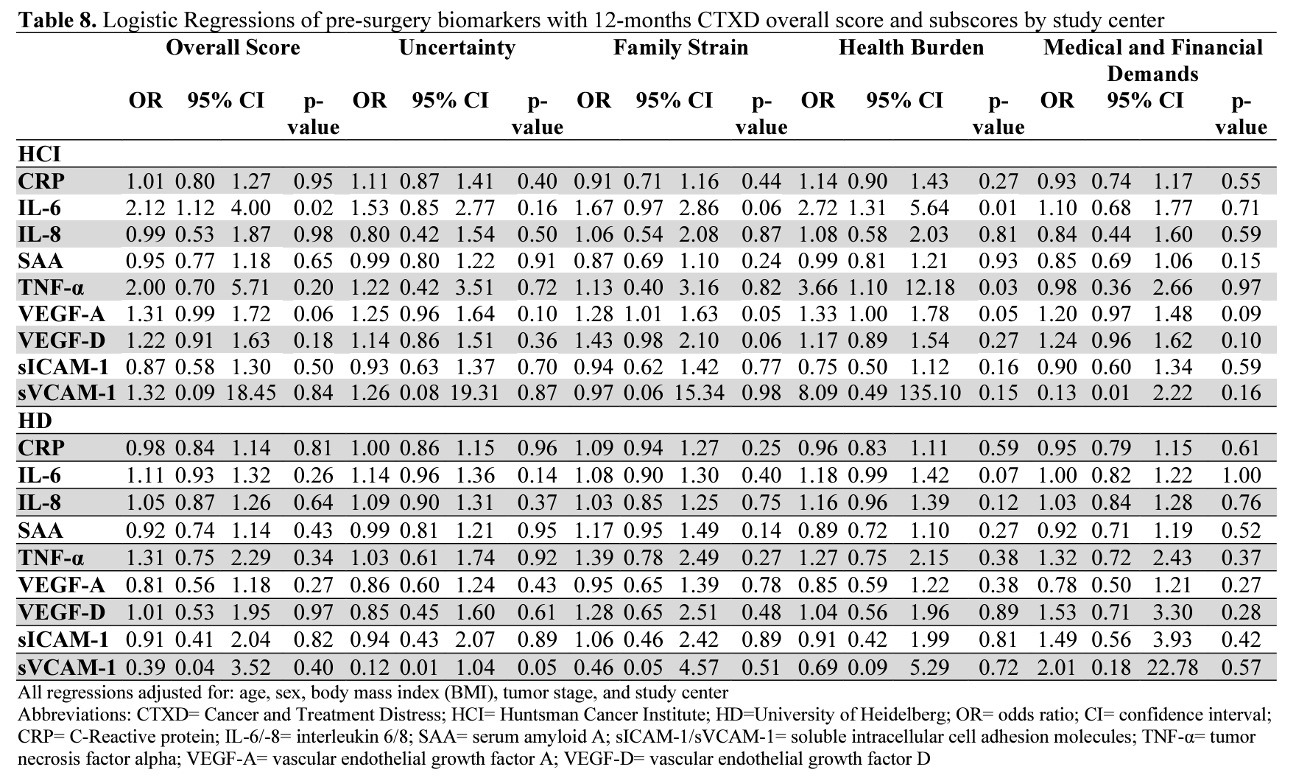
Pearson partial correlations are presented in Figures 1 and 2. A positive correlation coefficient (r)indicates that increased biomarker concentrations are correlated with distress and an inverse correlation coefficient indicates that increased biomarker concentrations are correlated with decreased distress. Any correlation with a p-value of<0.05 was considered statistically significant. We performed Bonferroni adjustment for multiple testing foreach of the investigated subscales. Logistic regressions adjusted for age, sex, BMI, tumor stage, and study center are presented in Table 7. Additional analyses stratified by study center are presented in Figures 3-4 and Table 8.
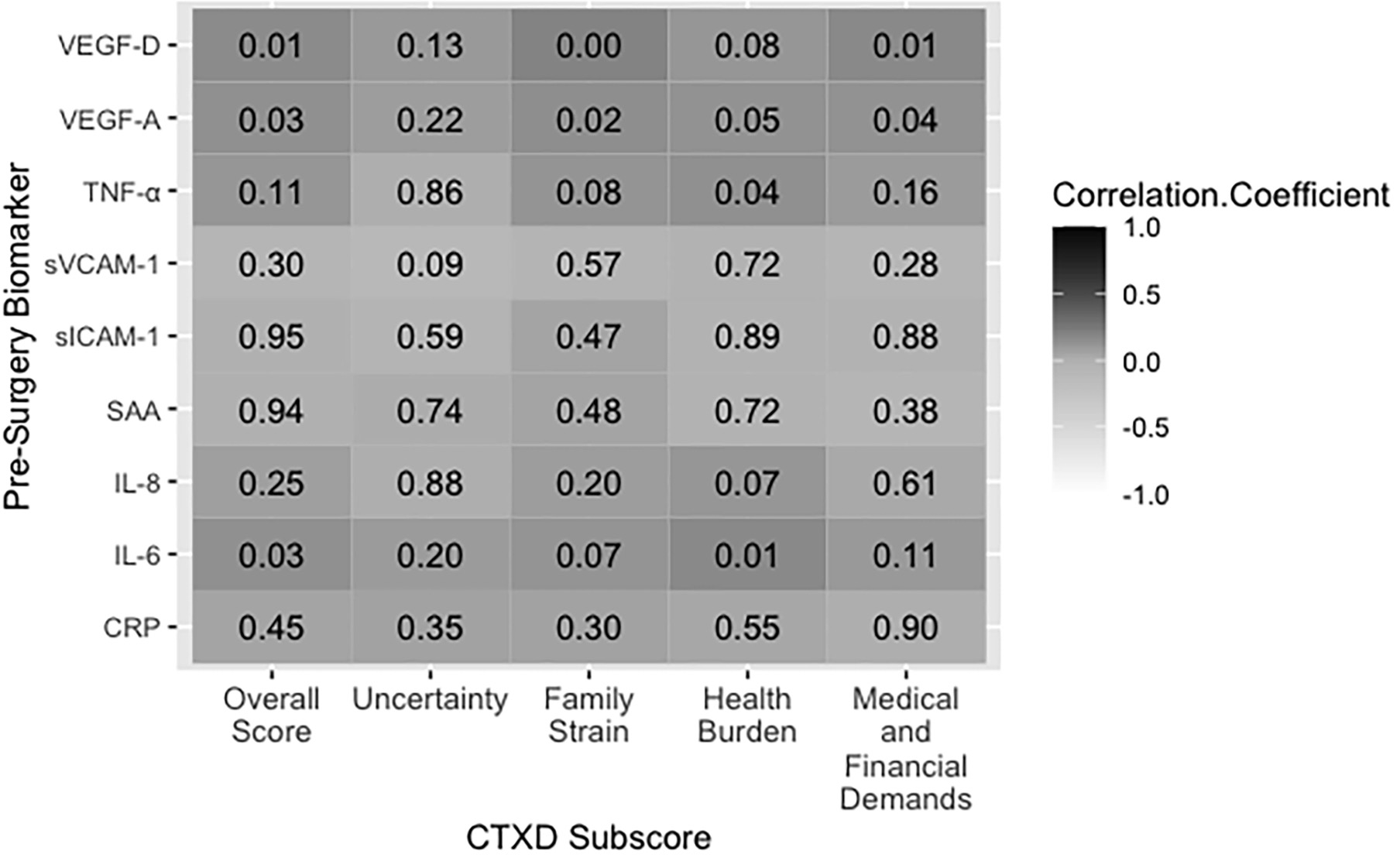
Figure 1. Pre-surgery biomarker concentrations correlated with the CTXD score 12 months after surgery. Dark grey indicates a positive correlation and decreasing grey value indicates decreasing correlation strength. The value in each box indicates the p value for significance. Abbreviations: CRP= C-Reactive protein; IL-6/−8= interleukin 6/8; SAA= serum amyloid A; sICAM-1/sVCAM-1= soluble intracellular cell adhesion molecules; TNF-α= tumor necrosis factor alpha; VEGF-A/-D= vascular endothelial growth factor A.
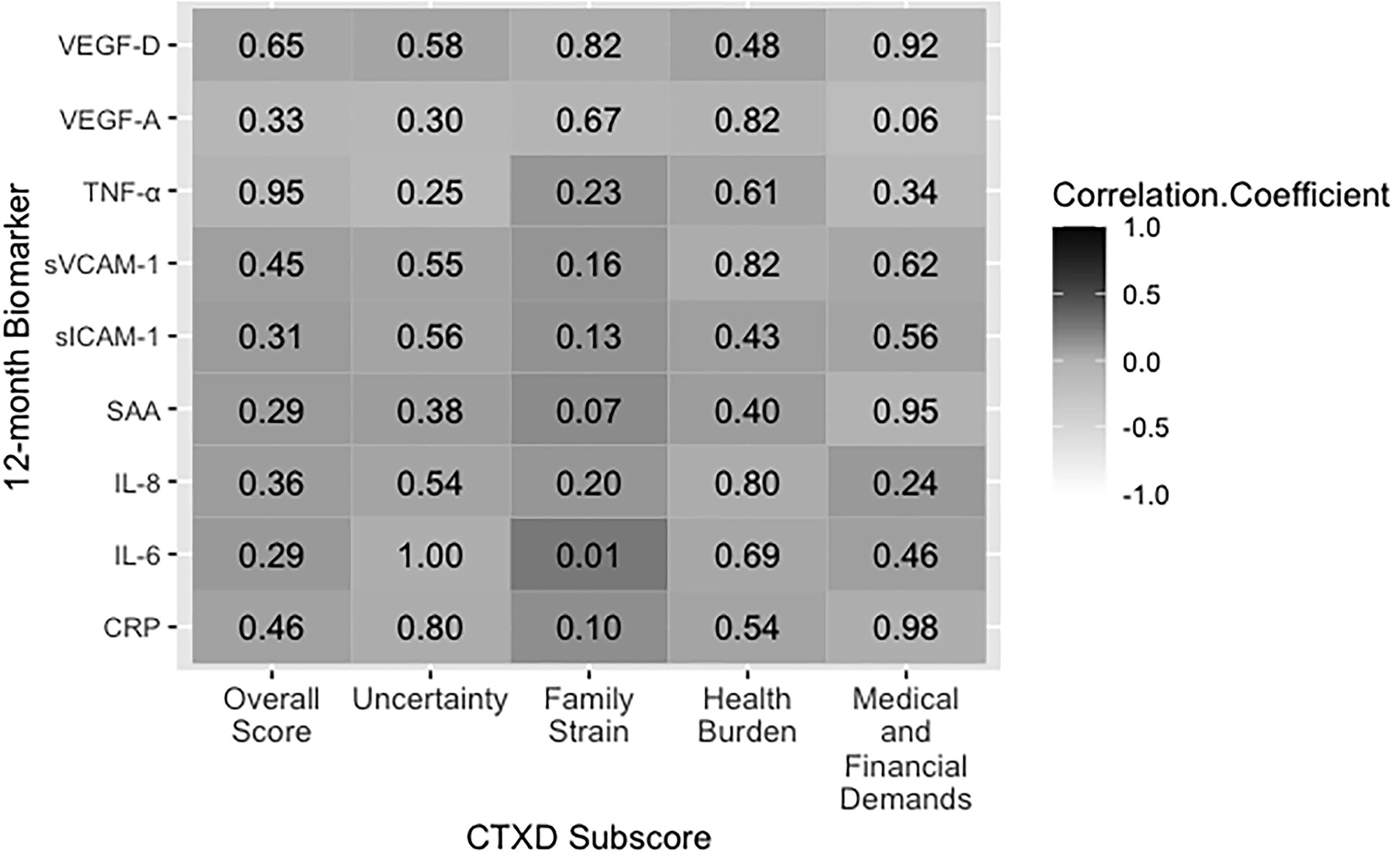
Figure 2. 12-month biomarker concentrations correlated with the CTXD score 12 months after surgery. Dark grey indicates a positive correlation and decreasing grey value indicates decreasing correlation strength. The value in each box indicates the p-value for significance. Abbreviations: CRP= C-reactive protein; IL-6/−8= interleukin 6/8; SAA= serum amyloid A; sICAM-1/sVCAM-1= soluble intracellular cell adhesion molecules; TNF-α= tumor necrosis factor alpha; VEGF-A/-D= vascular endothelial growth factor A/D.
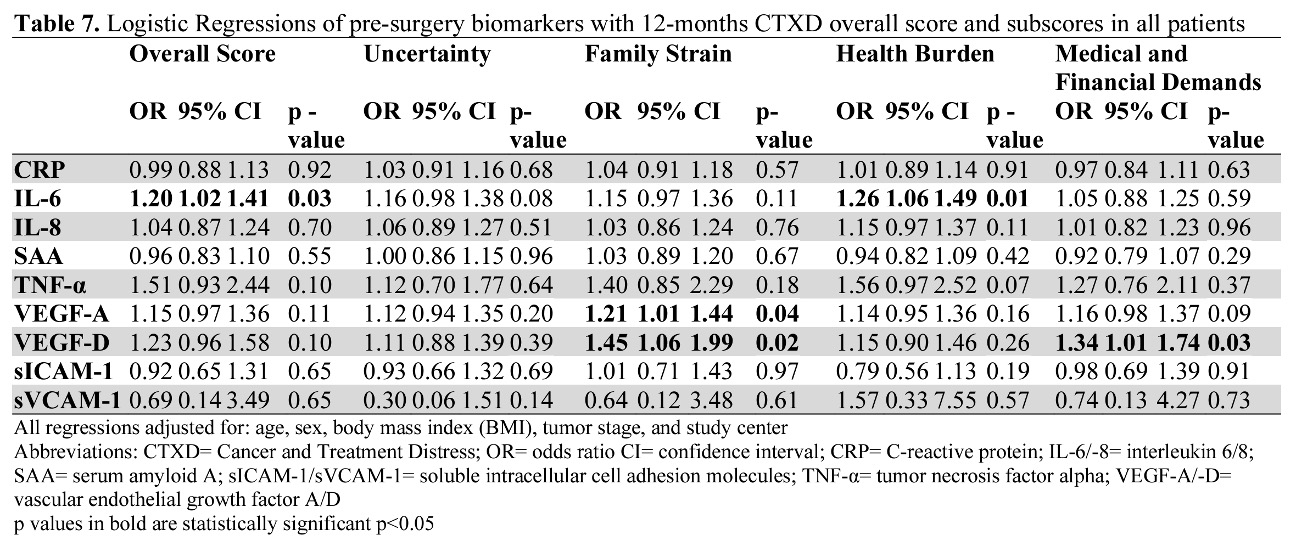
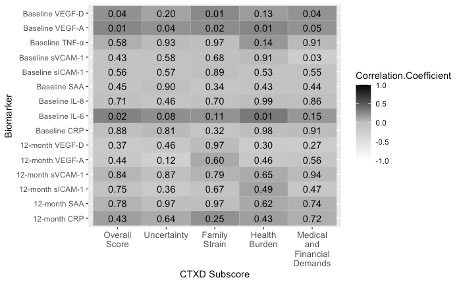
Figure 3. Pre-surgery and 12-month biomarker concentrations correlated with the CTXD 12 months after surgery at Huntsman Cancer Institute. Dark grey indicates a positive correlation and decreasing grey value indicates decreasing correlation strength. The value in each box indicates the p value for significance. Abbreviations: CRP= C-Reactive protein; IL-6/-8= interleukin 6/8; SAA= serum amyloid A; sICAM-1/sVCAM-1= soluble intracellular cell adhesion molecules; TNF-α= tumor necrosis factor alpha; VEGF-A= vascular endothelial growth factor A; VEGF-D= vascular endothelial growth factor D.
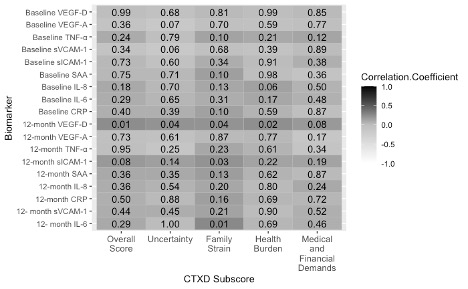
Figure 4. Pre-surgery and 12-month biomarker concentrations correlated with the CTXD 12 months after surgery at Heidelberg. Dark grey indicates a positive correlation and decreasing grey value indicates decreasing correlation strength. The value in each box indicates the p value for significance. Abbreviations: CRP= C-Reactive protein; IL-6/-8= interleukin 6/8; SAA= serum amyloid A; sICAM-1/sVCAM-1= soluble intracellular cell adhesion molecules; TNF-α= tumor necrosis factor alpha; VEGF-A= vascular endothelial growth factor A; VEGF-D= vascular endothelial growth factor D.
Overall Score
Pre-surgery IL-6, VEGF-A, and VEGF-D levels were correlated with an increase in overall CTXD score (r=0.14; p=0.03, r=0.14; p=0.03, r=0.16; p=0.02, respectively). Twelve months post-surgery, these correlations wereno longer significant, however IL-6, and VEGF-D correlations were in a similar direction (r=0.10 p=0.46, r=0.04; p=0.45, respectively). Doubling of pre-surgery IL-6 concentration predicted a 20% increased risk of having high distress (OR=1.20; CI=1.02–1.41; p=0.03) 12 months after surgery.
Uncertainty
Uncertainty was the highest observed subscore of the CTXD score across all patients and was endorsed by half of the survivors (52% with scores >0.90). No biomarkers were statistically significantly correlated withuncertainty at either time point. Pre-surgery IL-6 was marginally significantly associated with a 16% increased risk of high uncertainty (OR=1.16; CI= 0.98–1.38; p=0.08).
Family Strain
Pre-surgery VEGF-A and VEGF-D concentrations were correlated with increased family strain (r=0.15; p=0.02, r=0.20; p<0.01, respectively). Twelve months after surgery, IL-6 was correlated with family strain (r=0.25; p=0.01). The associations of VEGF-A and VEGF-D with family strain were significant in the logistic regression model. Doubling of pre-surgery VEGF-A concentration predicted a 21% increased risk of high family strain (OR=1.21; CI=1.01–1.44; p=0.04). Doubling of pre-surgery VEGF-D predicted a 45% increased risk of high family strain (OR=1.45; CI=1.06–1.99; p=0.02).
Health Burden
Pre-surgery IL-6, TNF-α, and VEGF-A concentrations were correlated with increased health burden (r=0.17;p=0.01, r=0.14; p=0.04, r=0.13; p=0.04, respectively). No biomarkers measured 12 months post-surgery were associated with health burden.
Pre-surgery IL-6 also predicted a 26% increased risk in health burden (OR=1.26; CI=1.06–1.49; p=0.01).
Medical And Financial Demands
Pre-surgery VEGF-A and VEGF-D were correlated with increased medical and financial demands (r=0.13;p=0.04, r=0.17; p=0.01, respectively). However, 12 months after surgery, the biomarkers were no longer associated.
Pre-surgery VEGF-D predicted a 34% increased risk of medical and financial demands (OR=1.34; CI=1.01–1.74; p=0.03).
Sensitivity Analysis
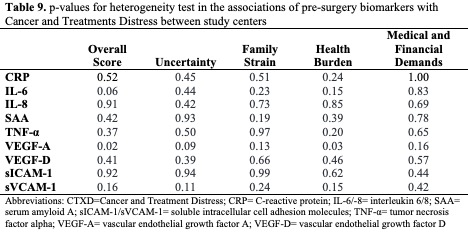
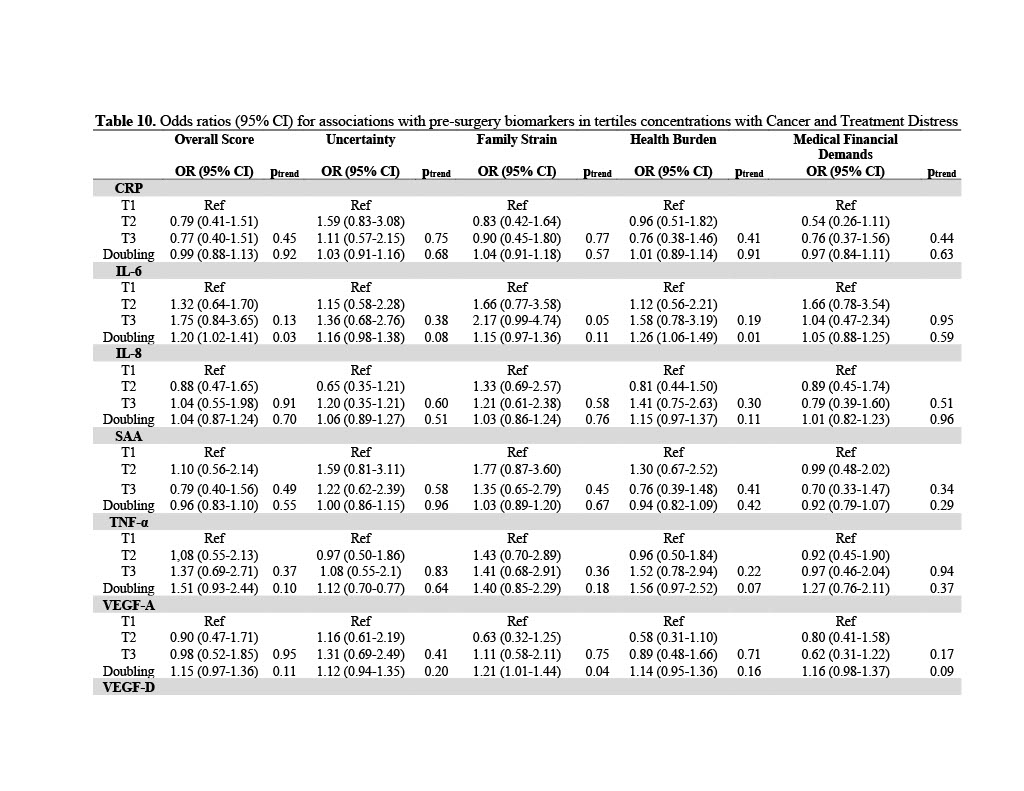
A test of heterogeneity in the associations showed comparable results across study centers, except for the association of pre-surgery VEGF-A with the overall score and the health burden score (pheterogeneity=0.02, pheterogeneity=0.03). Notably, VEGF-A was not significantly associated with the overall score or health burden in any of our statistical analyses. We did not observe heterogeneity in the associations of any biomarker with CTXD score in logistic regression models as shown in Table 9 (pheterogeneity>0.05). We additionally performed analyses stratified by tumor stage, neoadjuvant treatment, adjuvant treatment, and NSAID use. None of these variables were included in the final model. We further tested for linearity in the associations and investigated the p for trend in tertile analyses. Pre-surgery biomarkers had a dose response relationship with distress outcomes, as shown by the tertile analyses in Table 10.
Discussion
In this international, prospective study, the angiogenesis biomarkers VEGF-A and VEGF-D, were associated with distress in patients with CRC. VEGF-A and VEGF-D were correlated with increased overall distress, family strain, health burden (VEGF-D only) and medical and financial demands at the 12-month time point. Results from the logistic regression analyses showed that pre-surgery VEGF-A and VEGF-D concentrations predicted high family strain, and high medical and financial demands (VEGF-D only) 12 months after surgery. IL-6 was concurrently associated with overall cancer-related distress and subscales at pre-surgery and 12-months. These findings provide first evidence that angiogenesis and inflammation are associated with cancer- related distress 12 months after surgery. While the prediction is directional, the causality may be reverse. However, the interaction between pre-surgery biomarker levels and distress continues to demonstrate an impact at 12-months. Based on sensitivity tests, we conclude that the measured biomarkers were associated with distress outcomes independent of disease factors including tumor stage, tumor treatment, and study center. Angiogenesis may particularly interact with socioeconomic and stress disparities such as financial demands and family strain (35,36).
The associations between angiogenesis and cancer-related distress are consistent with a cross-sectional study that revealed women with ovarian cancer who reported high levels of social support had lower levels of pre-surgery VEGF (24). Our results showed that pre-surgery inflammation and angiogenesis biomarkers wereassociated with distress 12 months post-surgery. Longitudinal data of n=10,357 cancer-free adult participants with no evidence of depression at enrollment, revealed that increased CRP and white blood cell count (indicators ofinflammation) levels at enrollment, predicted new cases of depression 5 years later (7). Another study in the general population found that total white blood cell count is linked to an increase in depressive symptoms without evidence of a bi-directional relationship (17). In a systemic review on the effects of inflammation on depression, it was shown that if the peripheral immune system continues unabated, such as during systemic infections or cancer, the immune signaling can lead to exacerbation of symptoms and to the development of depression (8). The authors conclude that inflammation is an important biological event that might increase the risk of major depressive episodes, much like the more traditional psychosocial factors. While the above-mentioned studies investigate inflammation and distress in the general population, the directionality is consistent with our results in patients with CRC.
Pre-surgery CRP, an acute inflammation biomarker that has been associated with distress, was not associated with distress 12 months after surgery in our study (6,7,16). Acute inflammation, such as increased CRP levels, plays a more immediate role in the acute setting and does not predict distress one year after inflammation was measured.
Interestingly, increased concentrations of pre-surgery angiogenesis biomarkers are correlated with family strain, while 12 months post-surgery concentrations of other acute inflammation biomarkers are correlated with family strain. These results suggest that angiogenesis biomarkers are correlated with a longer-term or delayed distress response.
Cancer-related distress has well known impacts on quality of life in patients and long- term survivors. In response to this established fact, the National Comprehensive Cancer Network (NCCN) Distress Management and the NCCN Survivorship Guidelines each provide detailed recommendations for the assessment and treatment of cancer related distress, depression, anxiety, and post-traumatic stress symptoms (37). Similarly, the American Cancer Society guidelines specific to colorectal cancer survivors recommend at least annual surveillance and treatment of elevated distress (38). The National Cancer Institute provides guidance for health care professionals in screening and treating distress (39). Our results confirm that distress not only interacts with other quality of life outcomes, but also with biologic factors.
We have previously reported that pre-surgery angiogenesis may also affect long term survival (21). Pre-surgery VEGF-D has been associated with worse overall survival in patients with colon cancer (21), and a 3-fold increased risk of death in rectal cancer patients (21,40). The present study demonstrates that pre-surgery angiogenesis has an impact on cancer-related distress. In ongoing research, we are examining whether postoperative distress may interact with long term survival outcomes.
Our findings showed that patients had increased distress at our US-based site (HCI) compared to our Germany-based site (HD), specifically in the subscale’s uncertainty, health burden, and medical and financial demands. CTXD score averages, correlation coefficients, and ORs were generally stronger at HCI. The differences between study centers may be due to differences in health care systems between the two countries. Patients in the US are responsible for co-pays and high out-of-pocket expenses, which are nearly non-existent for patients in Germany, who are covered by universal health care (40). One study utilizing the CTXD score to determine risk factors for cancer-related distress in CRC survivorsrevealed that stable economic status pre-surgery is an important factor in preventing cancer-related distress over time (13). These differences may give rise to broad-based stress as seen with the higher levels of uncertainty, health burden, and medical and financial demands. The results from the heterogeneity test and the adjustment by study center in our analyses assures that the findings are applicable to patients across the study centers.
This study has several strengths and limitations. This study included a prospective longitudinal design, using a validated measure, CTXD score, to assess the association between inflammation and angiogenesis biomarkers with cancer-related distress. Since distress was not measured at baseline using the CTXD score, we cannot rule out ongoing associations of inflammation and distress throughout the course of treatment versus a direct effect of inflammation on distress. We used the electronic data warehouse at HCI to identify patients with a history of depression prior to their cancer diagnosis. We adjusted the main model for baseline depression, however no changes were observed in the presented odds ratios in comparison to the odds ratios before and after adjustment for depression. Unfortunately, these data are not available for the German cohort. Although our results show the prediction is directional, from pre-surgery to 12 months, the causality may be interactive. The critical point here is that biobehavioral processes do interact and may be amenable to biobehavioral interventions. The longitudinal design limits the risk of reverse causality and allows us to draw directionality conclusions. This study used validated subscales, adding specificity to the assessment. We did not assess pre-surgery cancer-related distress and could not control for pre-surgery distress in our analyses. IL-6, IL-8, and TNF-α data were only available pre-surgery for HCI patients, lowering the statistical power for these biomarkers. The magnitude of each correlation coefficient is relatively weak and may be due to small sample size. We do not have pathologic or molecular information of inflammatory indicators in primary tumors. A recent Nature publication highlights the interaction between systemic inflammation markers and primary tumor inflammation status. Zhao, et. al report that acute, primary tumor inflammation acts as a defense mechanism against infection or injury that can regulate anti-tumor immune responses. However, if the acute inflammatory reaction remains unresolved, it can transform into chronic inflammation generating an immunosuppressive tumor promotingenvironment (41). Our findings contribute evidence that this process also may interact with stress response factors reflected in perceived distress.
This is the first study to investigate a large comprehensive panel of inflammation and angiogenesis-related biomarkers in associations with cancer-related distress 12- months after surgery. In the present study inflammation and angiogenesis biomarkers measured at 12 months are both increased by distress and increase with distress. The underlying biology for these predicted effects remains to be illuminated through additional research. Importantly, this is the first study to show that pre-surgery angiogenesis biomarkers are correlated with and may predict cancer-related distress 12 months after surgery in CRC patients, though the underlying mechanism is unknown. Previous studies have revealed distress as a predictor of inflammation, whereas the present study shows that inflammation and angiogenesis can predict cancer-treatment related distress 12 months after surgery.
In conclusion, pre-surgery inflammation and angiogenesis-related biomarkers are associated with cancer-relateddistress 12 months after surgery. Based on our findings, interventions focused on lowering inflammation, such as diet, exercise, and anti- inflammatory medication, may improve cancer-related distress and quality of life in patients with CRC.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin 2021;71(1):7-33 doi 10.3322/caac.21654.
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin 2022;72(1):7-33 doi 10.3322/caac.21708.
3. Chad-Friedman E, Coleman S, Traeger LN, Pirl WF, Goldman R, Atlas SJ, et al. Psychological distress associated with cancer screening: A systematic review. Cancer 2017;123(20):3882-94 doi 10.1002/cncr.30904.
4. Riba MB, Donovan KA, Andersen B, Braun I, Breitbart WS, Brewer BW, et al. Distress Management, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2019;17(10):1229-49 doi 10.6004/jnccn.2019.0048.
5. Albrecht TA, Rosenzweig M. Management of Cancer Related Distress in Patients with a Hematological Malignancy. J Hosp Palliat Nurs 2012;14(7):462-8 doi 10.1097/NJH.0b013e318268d04e.
6. Pasco JA, Nicholson GC, Williams LJ, Jacka FN, Henry MJ, Kotowicz MA, et al. Association of high-sensitivity C-reactive protein with de novo major depression. Br J Psychiatry 2010;197(5):372-7 doi 10.1192/bjp.bp.109.076430.
7. Ernst M, Brähler E, Otten D, Werner AM, Tibubos AN, Reiner I, et al. Inflammation predicts new onset ofdepression in men, but not in women within a prospective, representative community cohort. Sci Rep 2021;11(1):2271 doi 10.1038/s41598-021-81927-8. Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness anddepression: when the immune system subjugates the brain. Nat Rev Neurosci 2008;9(1):46-56 doi 10.1038/nrn2297.
9. Tynan RJ, Weidenhofer J, Hinwood M, Cairns MJ, Day TA, Walker FR. A comparative examination ofthe anti-inflammatory effects of SSRI and SNRI antidepressants on LPS stimulated microglia. Brain Behav Immun 2012;26(3):469-79 doi 10.1016/j.bbi.2011.12.011.
10. Sacre S, Medghalchi M, Gregory B, Brennan F, Williams R. Fluoxetine and citalopram exhibit potentantiinflammatory activity in human and murine models of rheumatoid arthritis and inhibit toll-like receptors. Arthritis Rheum 2010;62(3):683-93 doi 10.1002/art.27304.
11. Adzic M, Brkic Z, Mitic M, Francija E, Jovicic MJ, Radulovic J, et al. Therapeutic Strategies forTreatment of Inflammation-related Depression. Curr Neuropharmacol 2018;16(2):176-209 doi 10.2174/1570159X15666170828163048.
12. Uher R, Tansey KE, Dew T, Maier W, Mors O, Hauser J, et al. An inflammatory biomarker as a differential predictor of outcome of depression treatment with escitalopram and nortriptyline. Am J Psychiatry 2014;171(12):1278-86 doi 10.1176/appi.ajp.2014.14010094.
13. Han CJ, Gigic B, Schneider M, Kulu Y, Peoples AR, Ose J, et al. Risk factors for cancer-related distress in colorectal cancer survivors: one year post surgery. J Cancer Surviv 2020;14(3):305-15 doi 10.1007/s11764-019-00845-y.
14. Batty GD, Russ TC, Stamatakis E, Kivimäki M. Psychological distress in relation to site specific cancermortality: pooling of unpublished data from 16 prospective cohort studies. BMJ 2017;356:j108 doi 10.1136/bmj.j108.
15. Chen Z, Zhang P, Xu Y, Yan J, Liu Z, Lau WB, et al. Surgical stress and cancer progression: the twistedtango. Mol Cancer 2019;18(1):132 doi 10.1186/s12943- 019-1058-3.
16. Kennis M, Gerritsen L, van Dalen M, Williams A, Cuijpers P, Bockting C. Prospective biomarkers of major depressive disorder: a systematic review and meta-analysis. Mol Psychiatry 2020;25(2):321-38 doi10.1038/s41380-019-0585- z.
17. Beydoun MA, Beydoun HA, Dore GA, Canas JA, Fanelli-Kuczmarski MT, Evans MK, et al. White blood cell inflammatory markers are associated with depressive symptoms in a longitudinal study of urban adults. Transl Psychiatry 2016;6(9):e895 doi 10.1038/tp.2016.180.
18. Miranda DO, Anatriello E, Azevedo LR, Cordeiro JFC, Peria FM, Flória-Santos M, et al. Elevated serumlevels of proinflammatory cytokines potentially correlate with depression and anxiety in colorectal cancer patients in different stages of the antitumor therapy. Cytokine 2018;104:72-7 doi 10.1016/j.cyto.2017.09.030.
19. Andersen BL, Goyal NG, Weiss DM, Westbrook TD, Maddocks KJ, Byrd JC, et al. Cells, cytokines, chemokines, and cancer stress: A biobehavioral study of patients with chronic lymphocytic leukemia. Cancer 2018;124(15):3240-8 doi 10.1002/cncr.31538.
20. Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, Cole SW. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol 2011;29(26):3517-22 doi 10.1200/JCO.2011.36.1154.
21. Ose J, Gigic B, Hardikar S, Lin T, Himbert C, Warby CA, et al. Presurgery Adhesion Molecules andAngiogenesis Biomarkers Are Differently Associated with Outcomes in Colon and Rectal Cancer: Results from the ColoCare Study. Cancer Epidemiol Biomarkers Prev 2022;31(8):1650-60 doi 10.1158/1055- 9965.EPI-22-0092.
22. Mousa L, Salem ME, Mikhail S. Biomarkers of Angiogenesis in Colorectal Cancer. Biomark Cancer 2015;7(Suppl 1):13-9 doi 10.4137/BIC.S25250.
23. Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med 2006;12(8):939-44 doi 10.1038/nm1447.
24. Lutgendorf SK, Johnsen EL, Cooper B, Anderson B, Sorosky JI, Buller RE, et al. Vascular endothelial growth factor and social support in patients with ovarian carcinoma. Cancer 2002;95(4):808-15 doi 10.1002/cncr.10739.
25. Ulrich CM, Gigic B, Böhm J, Ose J, Viskochil R, Schneider M, et al. The ColoCare Study: A Paradigm ofTransdisciplinary Science in Colorectal Cancer Outcomes. Cancer Epidemiol Biomarkers Prev 2019;28(3):591-601 doi 10.1158/1055-9965.EPI-18-0773.
26. Himbert C, Ose J, Nattenmüller J, Warby CA, Holowatyj AN, Böhm J, et al. Body Fatness, Adipose Tissue Compartments, and Biomarkers of Inflammation and Angiogenesis in Colorectal Cancer: The ColoCare Study. Cancer Epidemiol Biomarkers Prev 2019;28(1):76-82 doi 10.1158/1055-9965.EPI-18-0654.
27. Himbert C, Ose J, Lin T, Warby CA, Gigic B, Steindorf K, et al. Inflammation- and angiogenesis-relatedbiomarkers are correlated with cancer-related fatigue in colorectal cancer patients: Results from the ColoCare Study. Eur J Cancer Care (Engl) 2019;28(4):e13055 doi 10.1111/ecc.13055.
28. Ose J, Schock H, Tjønneland A, Hansen L, Overvad K, Dossus L, et al. Inflammatory Markers and Risk of Epithelial Ovarian Cancer by Tumor Subtypes: The EPIC Cohort. Cancer Epidemiol Biomarkers Prev2015;24(6):951- 61 doi 10.1158/1055-9965.EPI-14-1279-T.
29. Syrjala KL, Yi JC, Langer SL. Psychometric properties of the Cancer and Treatment Distress(CTXD) measure in hematopoietic cell transplantation patients. Psychooncology 2016;25(5):529-35 doi 10.1002/pon.3861.
30. Syrjala KL, Sutton SK, Jim HS, Knight JM, Wood WA, Lee SJ, et al. Cancer and treatment distress psychometric evaluation over time: A BMT CTN 0902 secondary analysis. Cancer 2017;123(8):1416-23 doi 10.1002/cncr.30454.
31. Jones SMW, Yi JC, Jim HSL, Loren AW, Majhail NS, Uberti J, et al. Age and gender differences in financial distress among hematopoietic cell transplant survivors. Support Care Cancer 2020;28(9):4361-71 doi 10.1007/s00520-019- 05291-1.
32. Kuba K, Esser P, Scherwath A, Schirmer L, Schulz-Kindermann F, Dinkel A, et al. Cancer-and-treatment-specific distress and its impact on posttraumatic stress in patients undergoing allogeneic hematopoietic stem cell transplantation (HSCT). Psychooncology 2017;26(8):1164-71 doi 10.1002/pon.4295.
33. Syrjala KL, Walsh CA, Yi JC, Leisenring WM, Rajotte EJ, Voutsinas J, et al. Cancer survivorship care for young adults: a risk-stratified, multicenter randomized controlled trial to improve symptoms. J Cancer Surviv 2021 doi 10.1007/s11764-021-01105-8.
34. Nørskov KH, Yi JC, Crouch ML, Fiscalini AS, Flowers MED, Syrjala KL. Social support as a moderator of healthcare adherence and distress in long-term hematopoietic cell transplantation survivors. J Cancer Surviv 2021;15(6):866-75 doi 10.1007/s11764-020-00979-4.
35. Zhang J, Ye ZW, Townsend DM, Hughes-Halbert C, Tew KD. Racial disparities, cancer and response to oxidative stress. Adv Cancer Res 2019;144:343-83 doi 10.1016/bs.acr.2019.03.012.
36. Muscatell KA, Brosso SN, Humphreys KL. Socioeconomic status and inflammation: a meta-analysis. Mol Psychiatry 2020;25(9):2189-99 doi 10.1038/s41380-018-0259-2.
37. Donovan KA, Deshields TL, Corbett C, Riba MB. Update on the Implementation of NCCN Guidelines for Distress Management by NCCN Member Institutions. J Natl Compr Canc Netw 2019;17(10):1251-6 doi 10.6004/jnccn.2019.7358.
38. El-Shami K, Oeffinger KC, Erb NL, Willis A, Bretsch JK, Pratt-Chapman ML, et al. American Cancer Society Colorectal Cancer Survivorship Care Guidelines. CA Cancer J Clin 2015;65(6):428-55 doi 10.3322/caac.21286.
39. PDQ Cancer Information Summaries. 2002.
- Ridic G, Gleason S, Ridic O. Comparisons of health care systems in the United States, Germany and Canada. Mater Sociomed 2012;24(2):112-20 doi 10.5455/msm.2012.24.112-120.
- Zhao H, Wu L, Yan G, Chen Y, Zhou M, Wu Y, et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther 2021;6(1):263 doi 10.1038/s41392-021-00658-5.

