John and Marcia Price College of Engineering
21 Temperature-Dependent Performance Analysis of Marketed Low-Fluorinated Nordic Ski Glide Waxe
Clara Kramer
Faculty Mentor: Jeffrey S. Bates (Materials Science and Engineering, University of Utah)
In the ski industry, the performance mechanisms of ski glide wax are a heavily debated topic. Waxes are applied to the bottom of polymer ski bases to modify the performance properties, oftentimes for use at certain optimum temperatures or snow conditions. There are no firm industry claims as to why certain base coatings perform the way that they do, and because of this, there is little published research supporting the performance mechanisms of base coatings. In this experiment, the temperature dependence performance of three main proposed mechanisms was tested: hydrophobicity, hardness, and the coefficient of friction. This was done using four common industry waxes, and the results showed that there is no correlation between the advertised use temperature of the wax and the performance of the proposed properties. This leaves the performance mechanisms continuously unknown for further research and provides a basis for future research and exploration.
Keywords: ski, ski wax, fluorinated compounds, human health, sustainability
Introduction
For hundreds of years, humans have used various coatings to improve the glide performance of skis. This began with the use of varying animal fats on wooden skis and eventually led to paraffin (alkane) wax compositions specially formulated to be applied to an ultra-high molecular weight polyethylene (UHMWPE) treated ski base [1]. Waxes are historically applied with the aid of heat, most recently a special heat iron designed for the purpose.
However, as wax has progressed, there are now wax coatings that can be applied by rubbing them into the base without the addition of heat or spraying a liquid wax onto the ski and letting it dry and cure to the base. The original purpose of wax on skis was to prevent the wooden ski base from absorbing water from the snow overcoming the hydrophilic nature of wooden skis. When ski bases developed further to be made using plastic base materials, the waxing continued, though the need for water absorption prevention was no longer present.
Ski waxes are sold by hundreds of companies worldwide, the most popular being Swix and Toko. Though the composition of each wax is not known due to industry patents, all ski waxes are made using a soft polymer base, with varying additives used to change the performance properties of the resulting ski base surface. The performance of skis due to the coatings that are applied has long been a debated topic in the skiing community. The waxing used on Nordic skis is extremely competitive, but the mechanisms by which this wax works are not fully understood.
It is generally believed that the application of wax aids with a temperature-dependent decrease in friction and an increase in hardness and hydrophobicity. Along with the physical performance properties that the wax provides, it also is used to protect the base material from abrasion by the snow crystals. Because skiwaxes are made of generally soft polymers, the temperature dependence of the coating is important to the performance of the wax on varying temperatures of snow [2]. Chemical additives are added to the paraffin wax to stabilize it and add varying properties. The performance properties of a material on snow are not only important for recreational uses such as skiing but are also of interest in other applications, such as tires or military equipment in cold-weather regions [3].
Today, the claimed highest-performing waxes are fluorinated waxes, or wax products that contain fluorine, which may increase hydrophobicity due to its size and electrostatic polarity. Today, ski bases are petroleum-based products with a chemical composition of CH3–(CH2)n– CH3, where n ranges from around 10-80 units,determining the final chain length. Fluorinated wax compounds are comprised of dispersed fluorinated copolymer blocks throughout the chain (CH3–(CH2)m–(CF2)m′–CF3) where m is around 15, and m’ is between 2 and 20 [4, 5].
From figure 1, it can be seen that the copolymer formed remains majority hydrocarbon- based, with smaller segments of fluorinated carbons throughout the chain.
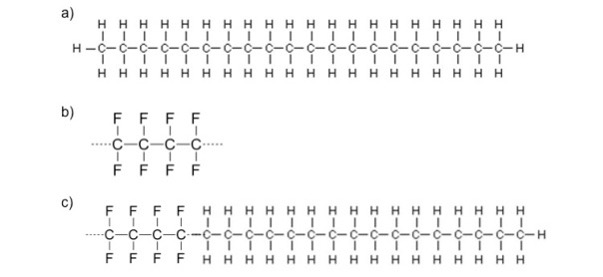
Figure 1: Starting with a hydrocarbon (a) and a fluorocarbon (b), a block polymer is formed for optimum ski performance.
Though many people claim that fluorinated compounds as an additive increase ski performance dramatically,many research studies prove this is not necessarily the case [6, 7]. Because of the number of influences on the final performance, it is difficult to determine which of these are due to the fluorinated compounds themselves.
Recently, it was found that ski wax usage and application contributed to a quantifiable amount of perfluoroalkyl (PFA) substances in the watershed and the soil near popular ski areas [4]. The use of PFAs inthe ski wax industry was banned in 2020 by the FIS World Ski Federation, both from all competition as well as from manufacturing and sales [8, 9]. PFAs are substances that are often desired for their high hydrophobicity and are often used in products such as grease, lubricants, packaging, fire repellents, and ski waxes. Though the hydrophobicity of PFA chemicals is a desired trait in these applications, there are alsomany downsides to these chemicals.
PFA substances pose many human health dangers. They have been shown to cause cancer development, inhibit immune function, and hinder neurodevelopment [10]. Additionally, once these substances are in the water or soil, they are extremely difficult to remove. They travel with the water, meaning that if a food source uses the water, the PFAs can be passed on to humans through eating the food, whether it be a plant or animal, not just by drinking the water directly. For ski wax technicians, this poses a very direct threat, asthese chemicals are released into the air during the waxing process. Ski wax technicians are exposed to exceedingly high levels of PFA chemicals during the wax season, and though it does decrease in the summer, time spent as a professional wax technician leads to a buildup of these chemicals in the bloodstream [11]. This eventually led to the banning of PFA- waxes for use in winter sports applications, leading to many companies and researchers looking to find a way to recreate the properties that these waxes were able to provide. The first step in this process is to determine the reasons why the performance of skis changes with different coatings and at different temperatures. There are many hypotheses as to what the cause of the temperature-dependent properties might be, which is prevalent in ski science. Because of the current lack of research, most of the research done today is seminal and groundbreaking in some way. Many mechanisms have been proposed to explain the performance of skis bases on snow including the effect of self-induced vibrations of the ski, roughness, and hardness [12, 13]. The three most accepted mechanisms for ski performance today are hardness, hydrophobicity, and friction factor.
Previously, it was thought that the performance of skis was only due to the hydrophobic interactions betweenthe wax layer on the bottom of the ski and the snow. The hydrophobicity of a material is its ability to repel water due to the molecule’s polarity. This is measured by the contact angle, or the angle of the water against the surface. Original theories as to how ski performance worked said that a layer of water was formed due to the interactions between the wax and the snow. This layer of water in between the ski and the snow is what resulted in the glide that skis have. If this were true, the ski performance would be almost entirely due to hydrophobicity. Though it is still thought that hydrophobicity is an influencing factor in performance, it is not the main or sole reason [9, 14]. There are many other plausible reasons for the glide created by a polymer base on snow, and hydrophobicity is only one of the many mechanisms. The hardness of the ski base is thought to change the interactions that occur between the ski base itself andthe snow crystals. Depending on the humidity and roughness of the snow, the hardness impacts the ability of the ski to “smooth” the snow surface, as opposed to having deformation of the base material due to the snow abrasion. The hardness of waxes is dependent on the chain length of the wax polymer that is used [2]. Theapplication of the wax itself is also thought to have an impact on the resulting base hardness [15]. There are no industry standard testing methods for hardness, so for this paper, a Shore A hardness scale was chosen to be the most accurate method for soft polymers such as ski wax.
The friction factor of the ski on snow is a mechanism that has a much broader scope, as the reason behind the friction itself is unknown. A common friction testing method that is used in this experiment is the Stribeck testing method. Stribeck testing is a nonlinear velocity testing method, where the velocity is changed at a logarithmic rate, and the friction factor is measured as a function of the sliding speed. Because there are no current standardized ski performance testing methods, testing ski sliding on snow is very difficult [16]. Friction, like hydrophobicity, is thought to be dependent on the interactions between the ski base and the snow surface. Additionally, the abrasion of the ski base may be a contributing factor due to the breaking of the UHMWPE polymer chains [17].
All the factors listed above play a part in the performance, though each’s importance is unknown. The factors listed above likely play a part in the performance, though the importance of each is unknown. This experiment aims to test the relationship between the hardness, hydrophobicity, and friction factor with theoptimum use temperature of ski waxes that are currently sold in the industry. It was hypothesized that the properties of the waxes sold would follow the proposed mechanisms- higher hardness, lower friction, and higher hydrophobicity- at the usetemperature for each of the four waxes tested. From this study, it was found that this is not the case for the low-fluorinated ski glide waxes that are currently sold on the market.
Materials and Methods
For this experiment, four common low-fluorinated waxes were tested. These were Swix brand LF6X, LF7X, LF8X, and Universal glide wax (figure 2), used as obtained from the manufacturer. The waxes were applied to circular sintered UHMWPE base samples of diameter 60 millimeters (mm) with a ski wax iron at the recommended application temperature for each wax as noted on the packaging and in table 1. The sampleswere then scraped and brushed using a SWIX nylon wax brush. This was done following standard waxingprocedure, and all samples were scraped and brushed in the same manner, with the only differing variable being the wax iron temperature. The samples were then used as the ‘ski base’ surface for testing. The tests used were standardized testing methods developed by researchers at the University of Utah and are noted below.

Figure 2: Swix LF6X glide wax, one of the four used in this experiment.
Each of these waxes is designed to work at an optimum temperature range, depending on the chemical properties of the wax (table 1). The exact composition of these ski waxes is unknown and protected by the manufacturing companies. The tests were run at either two different temperatures or a range oftemperatures, and all temperatures stated to be run at room temperature were run at about 18.5°C.
| Ski Wax Name | Optimal Performance Temperature | Application Iron Temperature |
| LF 6X | -10°C to -5°C | 145°C |
| LF 7X | -8°C to -2°C | 140°C |
| LF 8X | -4°C to 4°C | 130°C |
| Universal | *all | 120°C |
Table 1: Waxes used in the study alongside their ideal usage and application temperatures.
*Note that Universal glide wax did not clarify a use temperature, as it is for all snow conditions.
i. Hardness Testing
A Shore-A durometer (figure 3) was used to test the hardness of the wax at various temperatures. The hardness was taken on the waxed samples, as well as directly on the wax block. The shore values were taken at consequent increments of 5 seconds, to account for the softness of the material and the deformation over time. The temperatures at which hardness measurements were taken were at room temperature and 2 degrees Celsius. For analysis purposes, the temperatures at a time of 10 seconds were used to maintain a constant time measurement of hardness.

Figure 3: Shore A durometer testing method being tested directly on the wax block. The measurements weretaken at varying times after applying the measurement probe to account for the softness of the material.
ii. Hydrophobicity Testing
The hydrophobicity of the waxed samples was taken using the contact angle of a drop of water when resting on the surface. This was measured using a Dropometer Surface Analysis System, and the contact angle measurements were found using the included software (figure 4). Hardness measurements were taken on samples at room temperature and 2 degrees Celsius. The water applied was at room temperature.
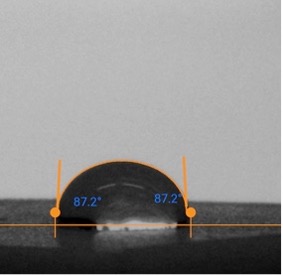
iii. Friction Testing
Friction factor testing was done using an Anton-Paar Tribometer. For testing the coefficient of friction, two main tests were run- Stribeck testing and a temperature- dependent steady-speed test. The test was run on a circular sample of prepared ski base (diameter 60mm) and was run using testing pegs made of High-Density Polyethylene (HDPE). For both test methods, a normal force of 5 Newtons was used. For the Stribeck test, the samples were run following a standardStribeck run pattern- a speed logarithmically increased from 1E-8m/s to 1m/s. This test was run at room temperature.
The second coefficient of friction test run was a temperature-dependent test at a steady velocity. The test was run at a velocity of 1 m/s over temperatures of -30 degrees Celsius to 10 degrees Celsius. The temperature changes occurred over around 10 hours, with a wait time at each single temperature to ensure that the correct temperatures were reached at each stage of the test. The friction was continuously tested to avoid overcoming static friction from intermittent stops. For both tests, water was applied to the wax surface to add a level of lubrication before beginning the test.
For each of the test methods used, at least 3 samples of each wax and temperature were tested. The averages were taken of the data to be analyzed. Data was analyzed and graphed using Anton Paar software and Python data analysis programming.
Results and Discussion
i. Hardness Testing
The analyzed hardness testing results can be seen in figure 5. It was hypothesized that the hardness of each wax would increase for the temperatures at which the wax was recommended for use. As shown in the results, the Universal wax increased in hardness at a cold temperature, which was notnecessarily expected, though following the properties of polymers, the hardness will increase naturally with a decrease in temperature.
For the LF8X wax, the hardness of the wax at cold temperatures was significantly harder than the room temperature wax. This does not align with the claim of higher hardness at performancetemperatures but can be explained by the thermal properties of polymers. Polymers are softer materials at higher temperatures, though these results do not support hardness being a key factor in temperature-dependent performance.
The LF7X wax performed as expected. This wax is more of a cold-weather wax, and so an increasein hardness with a decrease in temperature backed up the conclusion that hardness may be an influential factor in performance. The cold-weather wax (LF6X) was also the opposite of what was expected, though it also did not follow common polymer properties. The LF6X wax had increased hardness at room temperature as compared to the cold temperature tested. This further concludes that hardness is not a key player in the performance mechanisms of ski waxes.
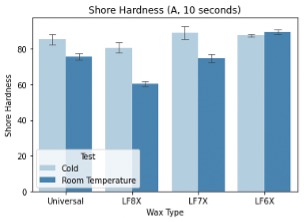
Figure 5: Shore-A hardness test readings at a time of 10 seconds. Tests were taken at two different temperatures, and results were averaged for analysis.
ii. Hydrophobicity Testing
The contact angle results obtained can be seen in figure 6. With a higher contact angle comes higher hydrophobicity which is one of the theorized performance mechanisms. The universal wax again performed as expected, with the contact angle being similar for both temperatures tested. This holds to the claim that, assuming hydrophobicity is an influential factor in performance, theUniversal ski wax will perform similarly at all temperatures.
Looking at the LF8X wax, the results compared to that of hardness in that they did not follow the proposed performance mechanism. The contact angle of the wax was larger at lowertemperatures, meaning that the surface was more hydrophobic at lower temperatures. The LF7X and LF8X waxes also directly contradicted this and had a higher contact angle at the room temperature sample. With the proposed performance mechanism being that a more hydrophobic surface increases the performance of the ski, the hydrophobicity of the surface should be maximized at the optimal usage temperature. For all temperature-specific waxes used in this study, the opposite was found, and they had a lower hydrophobicity at values closer to their usage temperatures.
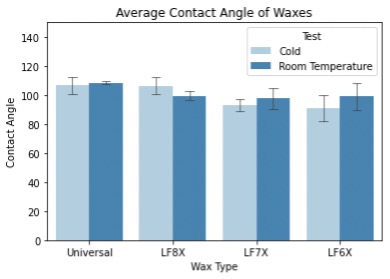
Figure 6: Average contact angle readings taken with the Dropometer contact angle measurement system.Tests were taken at two different temperatures, and results were averaged for analysis.
iii. Friction Testing
For all friction test graphs, the wax types follow that the lowest temperature wax is noted in the lightest color. Keys are provided on the graphs, but the correlation between optimal temperature andgraph color allows for easy visual analysis. Results were plotted using the Anton-Paar Rheometer software.
The Stribeck test results can be seen below in figure 7. At room temperature, testing an increase insliding velocity, it was found that LF6x had the lowest friction factor, and the universal wax had the highest friction factor. The increase in friction was proportional to the increase in optimal temperature, assuming that Universal can be used at temperatures higher than LF8X. Though this test was not temperature dependent, the results gave a baseline measurement of friction factor for all the waxes tested.
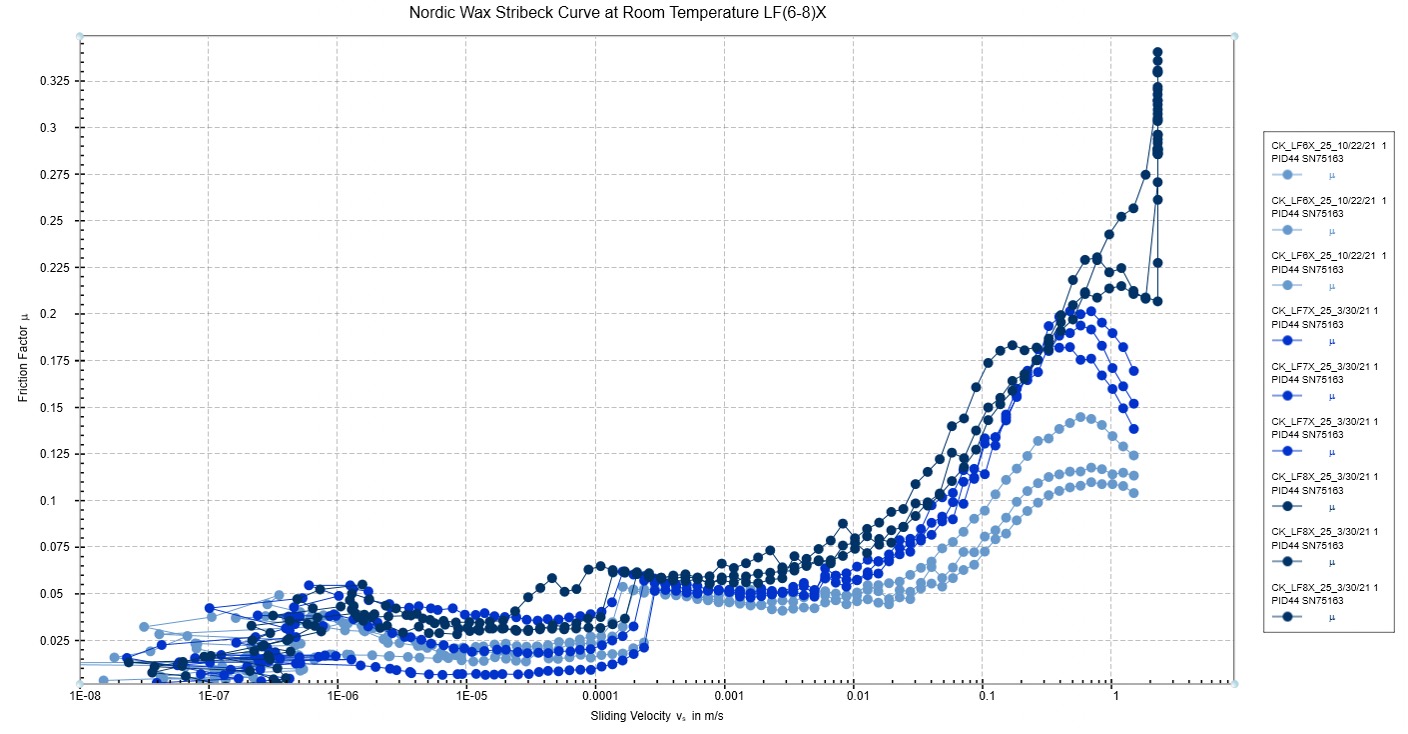
Figure 7: Stribeck curve friction results for four ski glide waxes. This test was run at room temperature and with a normal force of 5N.
The temperature-dependent effects of the friction factor on the frictional force are shown in figure 8. The Universal wax performed as expected and held a uniform friction force throughout the entire temperature range. This backs up the claims that this wax has the same (optimal) performance at all temperatures. The temperature-dependent waxes, however, performed in the same way that the previous tests have noted.The wax designed for use at the lowest temperature (LF6X) had the highest friction factor at lowtemperatures, and the friction factor was lowered in the higher temperature regime. The opposite was true for LF7X and LF8X, with these waxes having significantly lower friction factors at low temperatures and increasing the friction as the temperature rose. The point on the graph at which the waxes seemed to switch from their low-temperature regime performance to higher temperature performance occurred around -10°C. This temperature is noted as the lowest temperature for which we have a wax designed to perform, though the properties of the temperature-dependent waxes did not back up our hypothesis anywhere on thetemperature scale. These results are interesting in that many skiers and wax technicians believe that friction is theleading cause of ski performance. The results shown above show that this may not be the case, as the proposed use temperatures do not relate to a lower friction factor of the surface tested.
Figure 8: Temperature-dependent friction factor results tested on 4 wax types. This test was run over 10 hours to ensure a steady temperature scale.
Conclusion
The proposed mechanisms that were tested in this experiment were hardness, hydrophobicity, and the coefficient of friction. It had been previously proposed that an increase in hardness, increase in hydrophobicity, and decrease in friction factor led to optimized performance of a ski base on snow. Theresults explained above show that there is no correlation between the current claims that an increase in hardness and hydrophobicity alongside a decrease in friction factor led to optimized performance based on the marketed performance temperatures. Though the tests performed were not fully conclusive, they offered insight and background into some of the mechanisms that are thought to be key components of ski performance.
Future tests would be beneficial in backing up these claims, as well as testing other waxes on the market by other brands or meant for different temperature ranges. It would be beneficial to test the hardness and thehydrophobicity at temperatures that are closer to their usage temperatures, not at two temperatures that are higher than the usage temperatures. Another variablethat would be beneficial to test is the normal force applied during the friction tests. The force used in the tests run for this experiment was a 5 Newton normal force, significantly lower than that of a skier on a pair of skis. The speed at which these tests were run was also not reflective of the skier usage speed, and more advanced equipment will be needed to test higher speeds than those used in this experiment. Another future step in this test is to conduct field testing, to test the properties of the waxes in a more realistic environment on real snow.
Overall, it was found in this study that the proposed mechanisms of hardness, hydrophobicity and friction factor were not directly related to the optimum temperature for the waxes tested. The importance of thisfinding is that the claims made by current manufacturers are incorrect. This opens a door to a new topic of research that is essential for the progression of ski waxes, especially after the banning of PFA substances in 2020.
Footnotes
[1] L. Kuzmin and F. K. Fuss, “Cross-Country Skis.”
[2] S. Rohm et al., “Wear of ski waxes: Effect of temperature, molecule chain length and position on the ski base,” Wear, vol. 384, pp. 43-49, 2017.
[3] S. C. Colbeck, “A review of the processes that control snow friction,” 1992.
[4] G. L. Carlson and S. Tupper, “Ski wax use contributes to environmental contamination by per-andpoly-fluoroalkyl substances,” Chemosphere, vol. 261, p. 128078, 2020.
[5] F. Ludwig Sr, “Gas chromatography of petroleum-derived waxes and high- molecular-mass linearalcohols and acids,” Journal of Chromatography A, vol. 718, no. 1, pp. 119-129, 1995.
[6] I. Rogowski, J.-Y. Gauvrit, D. Léonard, and P. Lanteri, “Typology of the gliding waxes in cross-country skiing: Comparison between classifications based on the chemical composition and those based on the physical and physicochemical properties,” Cold regions science and technology, vol. 43, no. 3, pp. 140-149, 2005.
[7] I. Rogowski, D. Leonard, J.-Y. Gauvrit, and P. Lanteri, “Influence of fluorine-based additive contenton the physical and physicochemical properties of ski gliding wax,” Cold regions science and technology, vol. 49, no. 2, pp. 145-150, 2007.
[8] P. Svermova and M. Cernik, “Corporate social responsibility of companies
producing PFOA containing waxes for cross-country skiing,” Sustainability, vol. 12, no. 12, p. 5141, 2020.
[9] M. Scherge and T. Langer, “Are Fluorinated Ski Waxes Really the non plus ultra?,” Gliding, no. 3, pp. 14-19, 2020.
[10] M. Sunderland, X. C. Hu, C. Dassuncao, A. K. Tokranov, C. C. Wagner, and J. G. Allen, “A review of the pathways of human exposure to poly-and perfluoroalkyl substances (PFASs) and present understanding of health effects,” Journal of exposure science & environmental epidemiology, vol. 29, no. 2, pp. 131-147, 2019.
[11] Nilsson, A. Kärrman, H. Westberg, A. Rotander, B. Van Bavel, and G. Lindström, “A time trend study of significantly elevated perfluorocarboxylate levels in humans after using fluorinated ski wax,” Environmental science & technology, vo
[12] Koptyug, M. Bäckström, M. Tinnsten, and P. Carlsson, “Cross-country ski vibrations andpossible mechanisms of their influence on the free gliding,” Procedia Engineering, vol. 34, pp. 473-478, 2012.
[13] Kuzmin, “Investigation of the most essential factors influencing ski glide,” Luleå tekniska universitet, 2006.
[14] Kuzmin, “Interfacial kinetic ski friction,” Mittuniversitetet, 2010.
[15] Kuzmin and M. Tinnsten, “Hot glide wax treatment and the hardness of the ski running surface,” The engineering of sport, vol. 7, pp. 135-141, 2008.
[16] Márton and B. Lantos, “Control of mechanical systems with Stribeck friction and backlash,” Systems & Control Letters, vol. 58, no. 2, pp. 141-147, 2009.
[17] Nachbauer, P. Kaps, M. Hasler, and M. Mössner, “Friction between ski and snow,” The engineering approach to winter sports, pp. 17-32, 2016.

