Spencer Fox Eccles School of Medicine
74 Fully Magnetically Levitated Continuous Flow Left Ventricular Assist Device: Are We There Yet?
Tanner Frahm and Rami Alharethi
Faculty Mentor: Rami Alharethi (Internal Medicine, University of Utah)
Purpose
Despite improvements in the continuous flow (CF) durable left ventricular assist device (LVAD), hemocompatibility complications are still the major contributor to morbidity and mortality post implant. Recently, with the recall of HeartWare (HW) LVAD, the Heartmate 3 (HM3) became the only FDA approved LVAD as bridge to transplantation (BTT), bridge to decision (BTD) and destination therapy (DT). The purpose of this study is to compare the survival and rate of neurological and hemocompatibility complications including mucosal bleed and pump thrombosis in patients supported with Abbott HeartMate II (HM II), Medtronic HeartWare (HW) and Abbott (HM3) in our center.
Methods
The Utah Artificial Heart Program database was queried for patients supported with CF LVADs from 2004-2022. Patient hemocompatibility outcomes were retrospectively quantified. Rate ratios, confidence intervals, and significance testing were calculated with Poisson Tests for independence. Post-implant survival was calculated with Kaplan-Meier estimation. Survival was compared with log rank tests. 23 patients were excluded from adverse event analysis due to lack of follow-up. P-values < 0.05 were considered significant.
Results
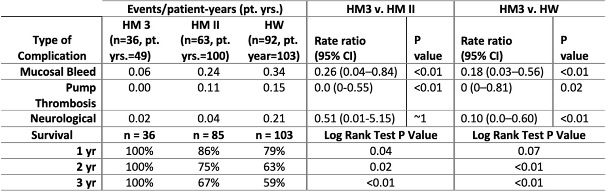
231 patients were implanted with CF LVADs at our center, HM3 n = 36, HM II n = 85, HW n = 103 with average age 56±13.8 yrs, 22% female and INTERMACS profile 3 being similar across all devices. The intent of therapy was comparable across the three devices. In comparison to both HM II and HW, the HM 3 was associated with statistically significant lower occurrence of almost all hemocompatibility complications and superior survival rates (see table).
Conclusion
In this large single-center study, the fully magnetically levitated CF LVAD outperformed the other generations of CF LVAD in both post-implant survival and rate of hemocompatibility complications. These results imply that the HM3 takes us a step closer towards the goal “there”.