College of Health
32 Effect of Increased Sleep Duration on Insulin Sensitivity and Sleep Regularity in Individuals with Habitual Short Sleep Duration (HSSD)
Elly LaMonte
Faculty Mentor: Christopher Depner (Health and Kinesiology, University of Utah)
Abstract
Background: As the field of sleep research grows, the correlation between inadequate sleep duration and debilitating health conditions becomes more apparent. While the majority of researchin this field has focused on sleep duration, recent findings have also stressed the importance of sleep regularity, especially in relation to the risk of chronic diseases, including diabetes.
Methods: We investigated the impact of a sleep extension intervention on sleep regularity and insulin sensitivity in adults with habitual short sleep duration (n=12). Participants in the Restoring Adequate Sleep Duration (RADS) study received interventions that aimed to increase their nightly sleep duration for four weeks. Participants’ insulin sensitivity (Matsuda Index) was measured by an oral glucose tolerance test, taken at both the baseline and post-intervention visits. Sleep regularity was determined by the Sleep Regularity Index (SRI) from wrist actigraphy data collected throughout the study.
Findings: Total Sleep Time was 5.3 ± 0.2 hours (mean ±SEM) at baseline and significantly increased 69.6 ± 10.32 minutes (mean ±SEM) during sleep extension. Matsuda Index was 5.96±0.64 (mean ±SEM) at baseline and significantly decreased 0.83 ± 0.3667 (mean ±SEM) after sleep extension. SRI was 75.8 ± 2.3 (mean ±SEM) at baseline and did not significantly change during after sleep extension.
Interpretation: Our original hypothesis predicted an increase in SRI, and a subsequent increase in Matsuda Index Values, as Total Sleep Time increased. Yet, preliminary analysis showed no change in SRI, a decrease in Matsuda Index, and an increase in Total Sleep Time. These findings show the sleep extension intervention successfully increased Total SleepTime without altering sleep regularity, suggesting Total Sleep Time and sleep regularity arepotentially independent dimensions of sleep health. Moreover, it is possible that the change to participants’ typical sleep-wake schedule caused an adverse shift in circadian rhythm that is underlying the negative changes in insulin sensitivity. These findings indicate interventions other than simple sleep extension may be required to improve sleep regularity and insulin sensitivity.
Introduction
Sleep duration, or the quantity of sleep received each night, has been emphasized as a public health crisis after studies were published revealing that 1 in 3 Americans receive less than adequate sleep on an average night [1]. Less than adequate sleep for adults is defined as less than 7 hours of sleep per night [1]. During initial research in the field, the main focus was to determine if sleep was “biologically necessary for life” [2]. Using experiments that focused on sleep deprivation and the related physiological consequences, researchers noticed drastic effects on participants who experienced radical changes in the duration of their sleep. This connection led researchers to draw the correlation between lack of sleep and the effects they hadnoticed, specifically focusing on studies that found rodents to die at an accelerated rate when sleep was significantly restricted[2].
Shift Work Sleep Disorder (SWSD) is an example of the consequence of not receiving adequate sleep regularly. Individuals who work nontraditional schedules, with part of their workday between the hours of 7:00 pm and 6:00 am are classified as shift workers [2]. As shift workers must be awake during the times that our circadian rhythms are tuning our physiology for sleep, SWSD can lead to “more sleep loss, excessive sleepiness, andinsomnia”. Medical complications include increased risk for cardiovascular disease, cerebrovascular eventsand stroke, obesity and metabolic disorders, GI complaints, and multiple forms of cancer. From a mentalhealth perspective, shift workers experience more psychiatric disease and greater psychosocial distress,including depression, anxiety, alcohol abuse, and work stress spillover as well as poorer quality of life”[2]. As a consistent lack of sleep is considered a public health epidemic, emphasis has been placed on remedying this issue before it negatively affects a larger population.
Sleep regularity– the consistency of an individual’s sleep and wake times throughout a night, week,month, or other pre-determined time frames– is a relatively new topic emerging in the field of sleep research. The Sleep Regularity Index (SRI) was first introduced in 2017 and further developed by Phillips, Fischer, and Klermann, researchers who aimed to develop a metric that could accurately measure different periods of sleep regularity. The SRI was a groundbreaking form of measurement because it allowed researchers to accurately compare an individual’s sleep regularity score and their likelihood of developing a chronic disease or increasing their risk of mortality. SRI is set apart from other forms of regularity measurements, such as intra-individual standard deviation (StDev), interdaily stability (IS), social jetlag (SJL), and composite phase deviation (CPD) [3] because it incorporates sleep and wakefulness across the entire 24-hour day.
While SRI is a relatively new form of sleep measurement, research has demonstrated the importance of studying SRI because it can “predict many health-related measures such as cardiac autonomic modulation, inflammation, metabolism, mental health, and performance and cognitive function” [3]. Previous to the development of SRI, many of these health outcomes were thought to be dependent on sleep duration and quality. This recent research, however, caused a large shift within the sleep and circadian rhythm research field, leading to a conversation on the impact of sleep duration versus sleep regularity. As this new area of research was further developed, researchers debated whether the research that had been put out in the past few years needed to be reevaluated in terms of the potential impacts of sleep regularity. In an article published in the esteemed SLEEP Journal in 2023, Windred and colleagues argued, “Sleep regularity is a stronger predictor of mortality risk than sleep duration: A prospective cohort study” [4]. This research study used over 10,000,000 hours of data collected from wrist actigraphy and compared the SRI scores from that data to mortality risks about 8 yearslater. Wrist actigraphy data was also used to calculate sleep duration on a nightly basis in addition to standard deviations of sleep-onset and sleep-wake times.
This study found that “sleep regularity and mortality exhibited a monotonic relationship, with higher sleep regularity predicting lower risk of mortality in Cox proportional hazards models. Individuals in the 80–100th sleep regularity percentiles had the lowest risk compared to the 0–20th percentiles… Sleep duration showed a non-linear U-shaped relationship with all-cause mortality in the minimally adjusted Cox model and a linear trend in relation to mortality in the fully adjusted model.
Longer sleep duration predicted a lower risk of mortality up to the 60–80th percentiles in the minimal model andup to the 80–100th percentiles in the fully adjusted model” [4]. The data illustrated that SRI was more strongly associated with overall mortality risk than sleep duration, illustrating the potential importance of sleep regularity to overall health.
The number of individuals diagnosed with diabetes, specifically Type II diabetes, has been steadily increasing worldwide over the past 5 decades [5]. This is alarming as Type II diabetes is linked to an increased risk of cardiovascular disease, lower limb amputation, blindness, kidney disease, and death [5]. As such, there is a critical need to discover and research potential risk factors for diabetes in an effort to help design new and more effective countermeasure strategies. As the sleep and circadian rhythm research field continues to grow, the connection between habitual short sleep duration (HSSD), and the risk of diabetes becomes increasingly clear [6]. More specifically, epidemiological data show people with HSSD are approximately 30% more likely to develop diabetes compared to people with adequate sleep duration [7]. Restricting sleep in healthy adults in the laboratory impairs insulin sensitivity to levels that are considered pre-diabetic [8]. Additionally, impaired insulin sensitivity is considered an independent risk factor for developing Type II Diabetes. However, data from tightly controlled laboratory studies in otherwise healthy adults does not directly translate to adults with HSSD and therefore the causal pathways underlying the link between HSSD and the risk of diabetes are not fullyunderstood, limiting the development of sleep-based interventions to help prevent diabetes. As diabetes can increase the risk of comorbidities such as cardiovascular disease, renal disease, and nerve damage, reducing the risk of diabetes can also lead to a reduced risk of other health afflictions and the potential for a longer, healthier life [1].
The primary outcome of this investigation was to determine the impact of a sleep extension interventionon insulin sensitivity and sleep regularity in the selected population of people with HSSD. This thesis aims to determine if there is a connection between increased Total Sleep Time (TST) and altered SRI and insulin sensitivity levels.
Methods
Participants: Participants were selected from a voluntary survey-based database and were required to meet rigorous inclusion-exclusion criteria in an effort to minimize outside factors from confounding results. To qualify for participation in the Restoring Adequate Sleep Duration study, individuals must be between 18 and 35 years old, have a BMI between 18.5 kg/m2 and 24.9 kg/m2, and receive less than
6.5 hours of sleep per night on average. Additionally, participants were unable to have certain medical and psychiatric conditions to be eligible. To date, 12 healthy participants (6 female; aged 20.1±2.5yr; BMI 20.6±2.69kg/m2 [mean±SD]) have completed data collection.
Study Design: Participants completed nine visits over the duration of this study (Figure 1). Study visits included a consent visit, in-laboratory baseline assessments, and overnight sleep monitoring visits. To assess insulin sensitivity levels, participants completed two oral glucose tolerance tests (OGTT) over the duration of this study. The first OGTT is completed at the conclusion of the participants’ baseline monitoring and the second OGTT is completed at the end of their sleep intervention. In order to determine SRI levels, participants complete multiple weeks of sleep measuring using a wrist actigraphy device, specifically, the Actiwatch Spectrum device. While data was collected for weeks 1-6 of the sleep extension intervention, I will only be using the first two weeks of data collected and the last two weeks of data collected for analysis. For the purpose of this thesis, I will only be analyzing data collected from participants who completed both the baseline and intervention segments up until the time of this report.
Data Processing: To process the sleep actigraphy data collected during this study the GGIR package in RStudio and other SRI-specific code in RStudio were used. After converting the analyzed actigraphy data into epoch-by-epoch data points, the “regularity.script” and “sleepreg” packages in RStudio convert the data from epoch-by-epoch sleep-wake data into a file containing only sleep-wake transitions and their corresponding UNIX times. Finally, this data file is run through the “GGIR for SRI” package in RStudio to return data that includes a participant’s SRI score in the form of a percentage between 0 and 100.
Statistical Analysis: To analyze the insulin sensitivity levels of each participant, the Matsuda Index, or MIV, (Figure 2) [9] utilizes the plasma glucose and insulin levels taken at the 0, 30, 60, 90, and 120- minute marks of the OGTT. The Matsuda Index was first introduced in 1999 in an effort to “evaluate whole body physiological insulin sensitivity from the data obtained by oral glucose tolerance test[s]” [9]. While the “normal value” for the Matsuda Index has been debated among sleep professionals, I will be using the value outlined in a comprehensive review of the Matsuda Index and other glucose measurement methods where a value of < 4.3 is indicates insulin resistance [10]. Changes in other variables between baseline and sleep extension were analyzed by linear mixed-effects models using the LME4 package in RStudio. Statistical significance was set at the α=0.05 level.
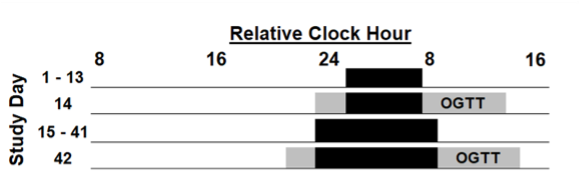
Figure 1: Protocol. Black bars indicate sleep opportunities. The y-axis represents the study day. Days 1- 13 represent the baseline segment and Days 15-43 represent the sleep extension segment.
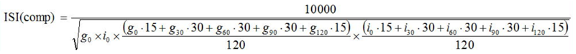
Figure 2: The equation developed by Dr. Matsuda and Prof. DeFronzo to quantify insulin sensitivity via the Matsuda Index. In this equation, g is equivalent to glucose levels, i is equivalent to insulin levels, and the subscript number is equivalent to the time after the OGTT was administered to the collection of the sample.
Results
Total Sleep Time (TST): TST was measured with wrist actigraphy devices with the use of self-reported sleep data to help determine daily bed and waketimes. TST was 5.3 ±0.2 hours (mean ±SEM) at baseline and significantly (p<0.05) increased 69.6 ± 10.32 minutes (mean ±SEM) during sleep extension (Figure 3). The maximum TST value was 7.24 hours (EXT) and the minimum TST value was 3.96 hours (BL).
Matsuda Index Values (MIV): Glucose and insulin data taken from oral glucose tolerance tests (OGTT) during both the baseline and post-intervention segments of the study were used to calculate the Matsuda Index values for each participant. The Matsuda Index quantifies a participant’s insulin sensitivity, a metric that can be used topredict risk of diabetes. MIV was 5.96 ±0.64 (mean ±SEM) at baseline and significantly (p<0.05) decreased 0.83 ± 0.3667 (mean ±SEM) after sleep extension (Figure 4).
Sleep Regularity Index (SRI): Utilizing data from the wrist actigraphy device worn during the baseline period and post-intervention period, an SRI percentage was calculated on a scale of 0-100 for each participant. SRI was 75.8 ± 2.3 (mean ±SEM) at baseline and 73.79± 2.1 (mean ±SEM) during sleep extension (Figure 5). The difference in SRI was found to not be statistically significant.
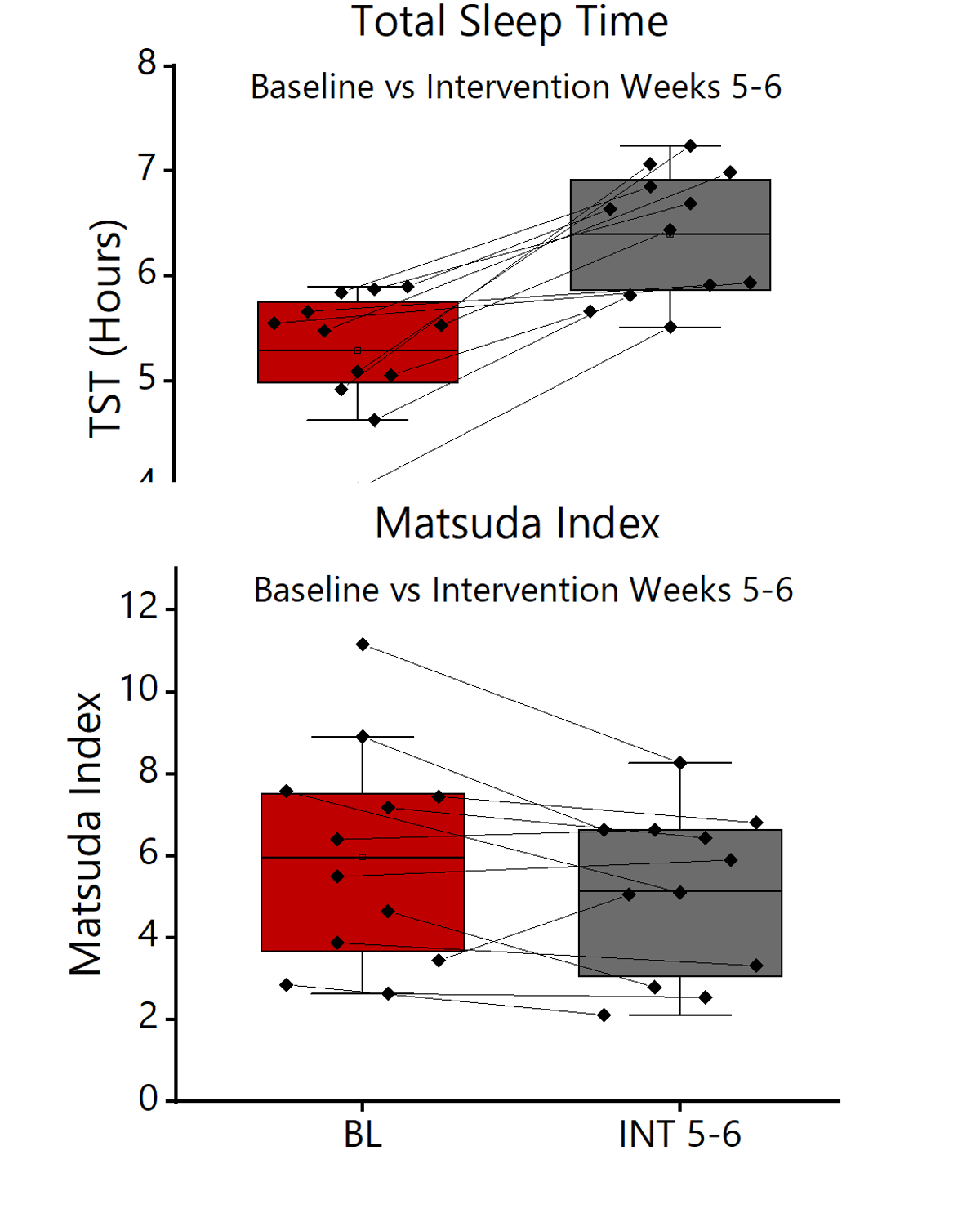
Figure 3 (Top): Total Sleep Time (TST) during baseline and intervention segments. (n=12). TST found to significantly (p<0.05) increase over the sleep extension intervention.
Figure 4 (Bottom): Matsuda Index during baseline and intervention segments. (n=12). MIV found to significantly (p<0.05) decrease over the sleep extension intervention.
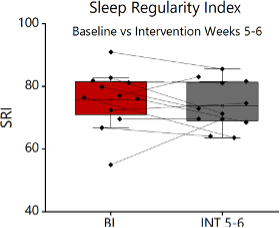
Figure 5: Sleep Regularity Index (SRI) values during baseline and intervention segments. (n=12). Nosignificant change found between SRI in baseline and intervention segments.
Discussion
The purpose of this thesis was to investigate if there was a significant connection between sleep duration and corresponding sleep regularity and insulin sensitivity levels in participants with HSSD. Based onthe results of our research at this time, a statistically significant increase in total sleep time (TST) was detected during the sleep extension intervention. This finding provides further evidence that sleep extension interventions are feasible to increase TST. Additionally, we detected a significant decrease in insulin sensitivity during sleep extension, opposite our hypothesis. As such, among adults with HSSD our findings suggest sleep extension did not have a statistically significant impact on sleep regularity.
Our hypothesis predicted an increase in insulin sensitivity values, a factor that could lead to a decrease in the likelihood of debilitating chronic diseases like diabetes. However, the Matsuda Index values found in this population were indicative of an increased risk of insulin resistance, a main risk factor for diabetes. As HSSDis considered a risk factor for diabetes, it will be important to follow-up on our findings in higher riskpopulations such as those with pre-diabetes. Although it is assumed that increasing sleep duration could help prevent diabetes, our data suggest this may not be the case, and more rigorous data in at-risk populations is critical before making clinical recommendations on potential sleep-based interventions
As the study is currently ongoing and data analysis has not been finalized, we have not yet determined the cause of an overall decrease in insulin sensitivity in response to sleep extension.
However, literature concerning experimental sleep restriction and impaired insulin sensitivity may help shedlight on potential causes. As seen with the SRI data, participants experienced a large shift in their normal sleep and wake times in an effort to increase overall sleep duration, which could have also had effects on their circadian clocks. These circadian rhythms are responsible for “the coordination of many daily processes, including the daily rhythm in human glucose metabolism. The central clockregulates food intake, energy expenditure, and whole-body insulin sensitivity” [11]. As participants’ sleep-wake times are altered, this could have led to a disruption in their circadian rhythms, possibly altering metabolic processes and hunger cues which could result in an increasedrisk of insulin resistance.
Additionally, previous reports have suggested, “that a critical amount of sleep is needed to benefit metabolic outcomes” [12]. While TST was found to significantly increase, there is the possibility that agreater amount of sleep was needed to lead to a positive impact on participants’ insulin sensitivity values. Regardless of the causal factors, our findings suggest sleep extension may not be the bestintervention to improve insulin sensitivity among adults with HSSD. For example, other interventions more specifically targeting circadian timing or SRI may have higher potential to improve healthoutcomes.
Despite a significant (p<0.05) increase in TST, no significant change was found in regards to SRI. The lack of connection between these two variables in our data suggests that while participants increased the amount of time they spent asleep, this was not in conjunction with increasing the regularity oftheir sleep-wake times. Thus, it is possible that sleep regularity may impact health outcomes by modulating mechanisms other than insulin sensitivity. However, because our data are preliminary anddo not have a wide range of SRI observations more work focused on the potential link between SRI andinsulin sensitivity is needed.
One of the potential limitations of this study is the small number of participants who have the study atthe time of this report. Only 12 participants had completed the necessary study components at the time of this report as the study and data analysis is currently ongoing. There is a possibility of internal and external invalidity as a result of the small sample size and the results of this study may change as more participants are included.
Conclusion
This study found that the sleep extension intervention significantly (p<0.05) increased TST. There wasalso a significant relationship between an increase in TST and a decrease in Matsuda Index values, which signify insulin sensitivity. We did find not a significant relationship between increased sleep duration and SRI scores.
The results of this report allude to a connection between our sleep extension intervention and impaired insulin sensitivity. However, these results conflict with the current literature, and the limitations of this particular study should be taken into account. As this study concludes, there should be in-depth analysis of thecomplete data set to ensure accurate conclusions. Additionally, measures of circadian timing should beinvestigated to determine if the sleep extension intervention caused adverse changes in circadian timing. If this is the case, that would inform the design of new interventions that focus on both sleep duration and circadian timing. Such findings would also suggest circadian timing may be a more important risk factor to target for reducing risk of diabetes.
Finally, an important next step for the field is to conduct similar investigations in higher risk populations such as individuals with obesity and older age.
Acknowledgements
I would like to thank Dr. Christopher Depner and Audrey Stegman for providing guidance throughout the thesis research and writing process. Additionally, assistance was offered by Dr.
Michael Gills during the development of this project. This work was supported by the University of Utah’s Undergraduate Research Opportunities Program and by the National Institute of Health (PI – C. Depner). Theseopinions, interpretations, and conclusions are my own and not necessarily endorsed by the funders.
References
[1] Centers for Disease Control and “Short Sleep Duration Among US Adults.” CDC, U.S. Department of Health & Human Services, 2014.
[2] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6859247/
[3] Fischer, , Klerman, E. B., & Phillips, A. J. (2021). Measuring sleep regularity: Theoretical properties and practical usage of existing metrics. Sleep, 44(10). https://doi.org/10.1093/sleep/zsab103
[4] Daniel P Windred, Angus C Burns, Jacqueline M Lane, Richa Saxena, Martin K Rutter, Sean W Cain, Andrew J K Phillips, Sleep regularity is a stronger predictor of mortality risk than sleep duration: A prospective cohort study, Sleep, 2023;, zsad253, https://doi.org/10.1093/sleep/zsad253
[5] Centers for Disease Control and (2023, April 24). What is diabetes?. Centers for Disease Control and Prevention. https://www.cdc.gov/diabetes/basics/diabetes.html
[6] Depner, C. M., Stothard, E. R., & Wright, K. P., Jr (2014). Metabolic consequences of sleep and circadian Current diabetes reports, 14(7), 507. https://doi.org/10.1007/s11892-014-0507-z
[7] Cappuccio, F. P., D’Elia, L., Strazzullo, P., & Miller, M. A. (2010). Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes care, 33(2), 414–420. https://doi.org/10.2337/dc09-1124.
[8] Depner, C. M., Melanson, E. L., Eckel, R. H., Snell-Bergeon, J. K., Perreault, L., Bergman, B. C., Higgins, A., Guerin, M. K., Stothard, E. R., Morton, S. J., & Wright, K. P., Jr (2019). Ad libitum Weekend Recovery Sleep Fails to Prevent Metabolic Dysregulation during a Repeating Pattern of Insufficient Sleep and Weekend Recovery Sleep. Current biology: CB, 29(6), 957–967.e4. https://doi.org/10.1016/j.cub.2019.01.069
[9] Matsuda, , & DeFronzo, R. (1999). Study on Index for Insulin Sensitivity. Matsuda index. http://mmatsuda.diabetes-smc.jp/english.html
[10] Gutch, , Kumar, S., Razi, S. M., Gupta, K. K., & Gupta, A. (2015). Assessment of insulin sensitivity/resistance. Indian journal of endocrinology and metabolism, 19(1), 160–164. https://doi.org/10.4103/2230-8210.146874
[11] Stenvers, D. J., Scheer, F. A. J. L., Schrauwen, P., la Fleur, S. E., & Kalsbeek, A. (2019). Circadian clocks and insulin resistance. Nature reviews. Endocrinology, 15(2), 75–89. https://doi.org/10.1038/s41574- 018-0122-1
[12] So-ngern A, Chirakalwasan N, Saetung S, Chanprasertyothin S, Thakkinstian A, Reutrakul Effectsof two-week sleep extension on glucose metabolism in chronically sleep-deprived individuals. J Clin Sleep Med. 2019;15(5):711–718. https://doi.org/10.5664/jcsm.7758

