Lennox-Gastaut Syndrome
Kobe Christensen
LENNOX-GASTAUT SYNDROME
Introduction
Lennox-Gastaut (LGS) is an uncommon pediatric epilepsy syndrome that makes up around 10% of childhood epilepsy. Lennox-Gastaut is characterized by the onset of multiple different seizure types, severe cognitive delay, and a distinct EEG pattern.
History
Lennox-Gastaut is named after the two physicians who first documented the disease. In the 1950’s, Dr. William Lennox described a particular EEG finding that would match an epilepsy syndrome that Dr. Henri Gastaut was studying in the mid 1960’s. Dr. Henri Gastaut described a childhood epilepsy with frequent tonic and absence seizures. Later it was found that both Dr. Lennox and Dr. Gastaut were studying the same epilepsy syndrome and thus it was coined Lennox-Gastaut syndrome.
Symptoms
Lennox-Gastaut is frequently associated with intractable epilepsy, severe cognitive impairment, and characteristic EEG.
Epilepsy
Patients with Lennox-Gastaut tend to present with multiple seizure types.
- Tonic
- Tonic-Clonic
- Atonic (Drop attacks)
- Myoclonic
- Atypical Absence (With characteristic EEG pattern)
Seizures in patients with Lennox-Gastaut are frequent in nature and tend to cluster making them at high risk for evolving into Non-convulsive status epilepticus (NCSE) with some studies showing an incidence of NCSE being over.
The average of seizure onset in patients with LGS is between 3-5 years of age. Almost all patients with an LGS diagnosis have an onset of seizures before 8 years of age.
Cognitive Impairment
Cognitive impairment before seizure onset is seen in roughly half of patients. Depending on the severity of the disease there may be a delayed onset of cognitive delay after the patient has their first seizure.
Characteristic EEG pattern
EEG in patients with LGS can be complex and difficult to read. Due to the multitude of seizure types, LGS seizure can present in several different ways which may make it difficult to differentiate the disease from others. However, in LGS there is a very characteristic EEG pattern known as atypical absence which helps to set it apart from others.
Atypical absence seizures are defined as slow (1.5-2.5 Hz) irregular polyspike and wave activity. It is important to note that atypical absence seizures differ from typical absence seizures in that they are slower (Typical absence seizures tend to be in the 3-5 Hz range) and are often asymmetric (Typical absence seizures are almost always generalized). Atypical absence seizures are NOT triggered by hyperventilation.
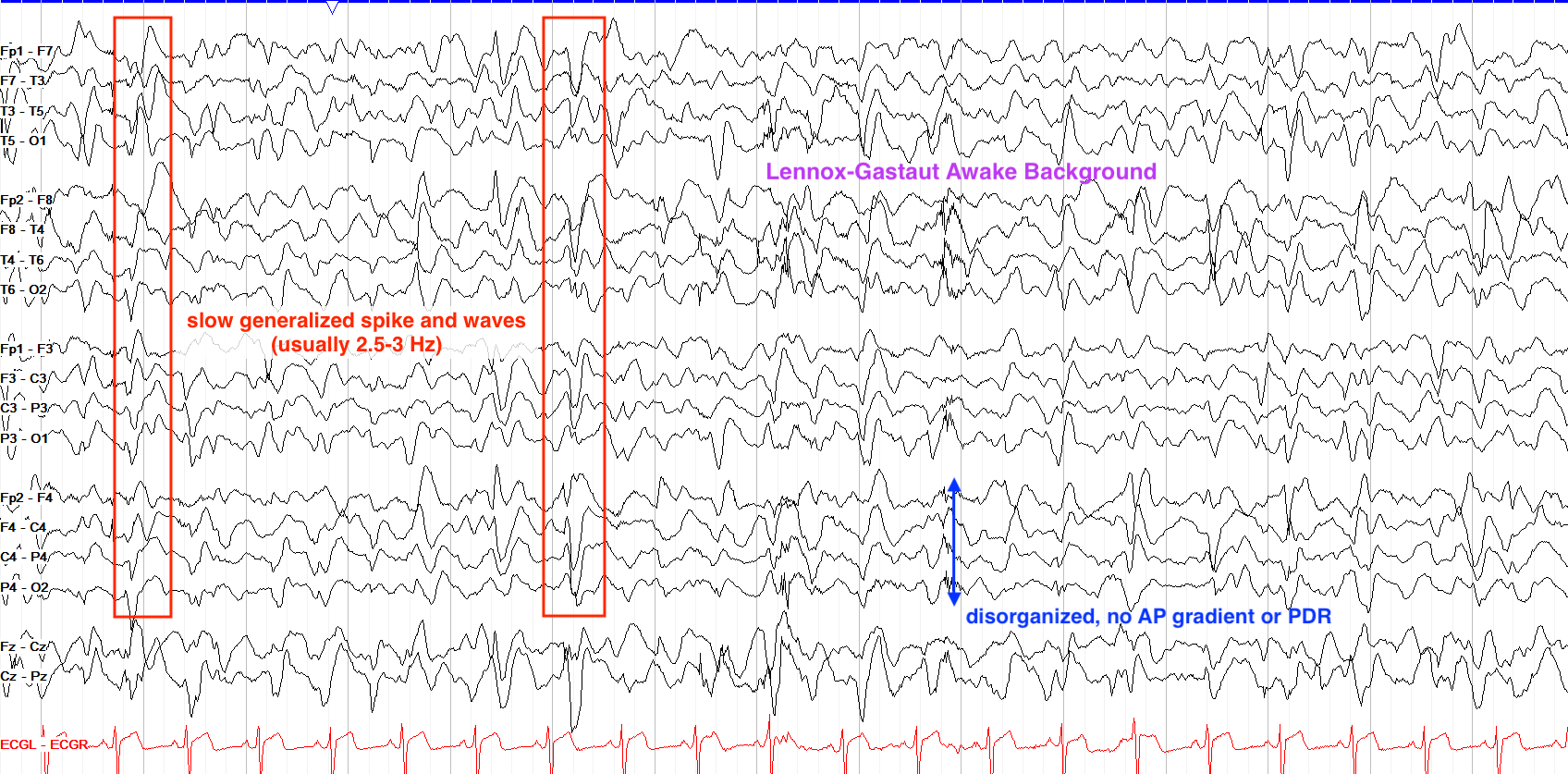
From “The Pediatric EEG”, by David Valentine M.D., 2020, (https://www.learningeeg.com/pediatric). Copyright 2020 by David Valentine
Figure 2.1 showing Lennox Gastaut awake background with 2-2.5 Hz generalized spike and wave.
Other patterns that can be seen in Ictal EEG of an LGS patient include:
- Rhythmic, high amplitude discharges in the high alpha-beta ranges seen in tonic seizures.
- Arrhythmic bursts of polyspike-wave discharges seen in myoclonic seizures.
Classifications of disease
LGS diagnoses typically fall into the following two categories: Secondary & Idiopathic
Secondary LGS:
This accounts for about 75% of all cases and describes a known primary cause. Any damage to the brain before or during birth can be considered a primary cause to LGS. These include:
- Infection
- Frontal lobe injury
- Tuberous Sclerosis
- Perinatal asphyxia
- Perinatal stroke
- Abnormal development
Idiopathic LGS
These account for the other 25% of LGS cases and have no known cause.
Linkage between Lennox-Gastaut & West Syndrome
An LGS diagnosis has been to known to frequently follow a myriad of other childhood epilepsy diagnoses. None are more common than the diagnosis of West Syndrome, as many LGS cases follow a diagnosis of West Syndrome despite no known cause for the linkage of the two.
Treatments & Prognosis
Long-term prognosis is generally unfavorable for patients with LGS. While mortality secondary to LGS is low, severe cognitive impairment and treatment resistant epilepsy still persists in the majority of patients. Patients with a history of West Syndrome also tend to have worse outcomes than those with idiopathic LGS.
Treatment options in LGS vary due to the multitude of seizure types. Antiepileptic drugs (AEDs) tend to be the mainline treatment for LGS but rarely does a single AED give complete relief of seizures. Valproic Acid (Depakote) and Lamotrigine (Lamictal) are the most commonly used AEDs in LGS. Other forms of treatment include the Ketogenic Diet, VNS, and surgical intervention. LGS patients are good candidates for corpus callostomy to help reduce the frequency of seizures, though it is rare for surgical intervention to be completely curative.
Key Takeaways
- 1.5-2.5 Hz Absence seizures are a common EEG finding in patients with LGS.
- LGS is likely to be secondary to some form of neurological damage before or during birth.
- There is a common progression of West Syndrome to Lennox-Gastaut.
- Valproic acid is the mainline treatment for LGS, followed by a variety of other AEDs as needed.
Objective 3: Demonstrate how the embryonic development of the brain is reflected in the structure of the spinal cord.
Objective 3 Video Lecture
Recall that the central nervous system is comprised of the brain and spinal cord. We’ll consider the brain later, but first we will study the spinal cord.
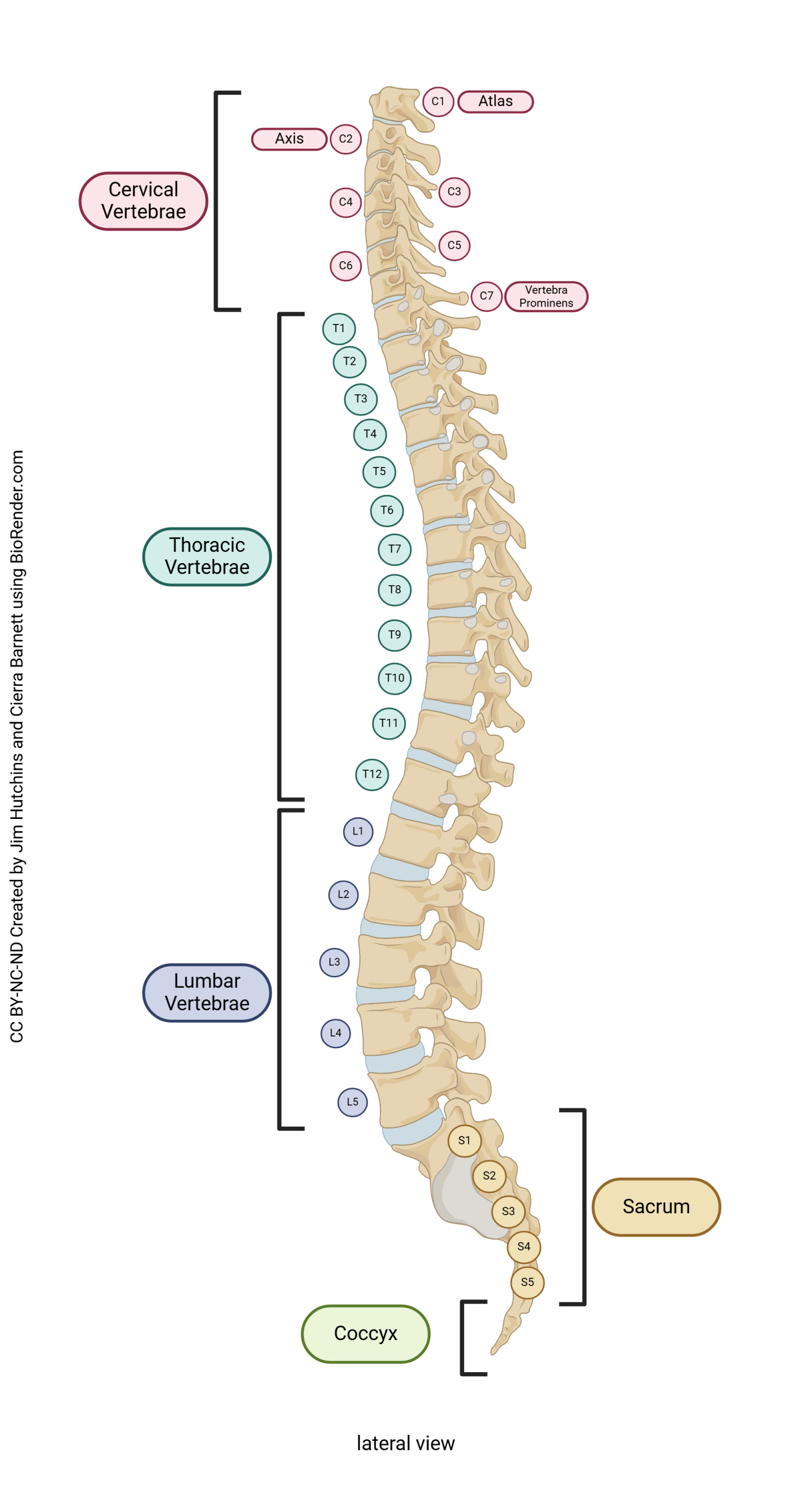
Take time now to review the anatomy of the vertebral column (Unit 9). Recall that there are 7 cervical vertebrae, 12 thoracic vertebrae, 5 lumbar vertebrae, 5 fused sacral vertebrae, and 3-4 fused coccygeal vertebrae. The vertebrae are numbered from top to bottom, with a prefix indicating cervical (C1-C7), thoracic (T1-T12), lumbar (L1-L5), or sacral (S1-S5). These will surround the spinal cord, but neurologists also define spinal cord segments which bear a relationship to the vertebrae nearby.
The spinal cord is about the diameter of your little finger at its largest (where it connects to the brain) and tapers to a point at its end (the filum terminale, “terminal thread”).
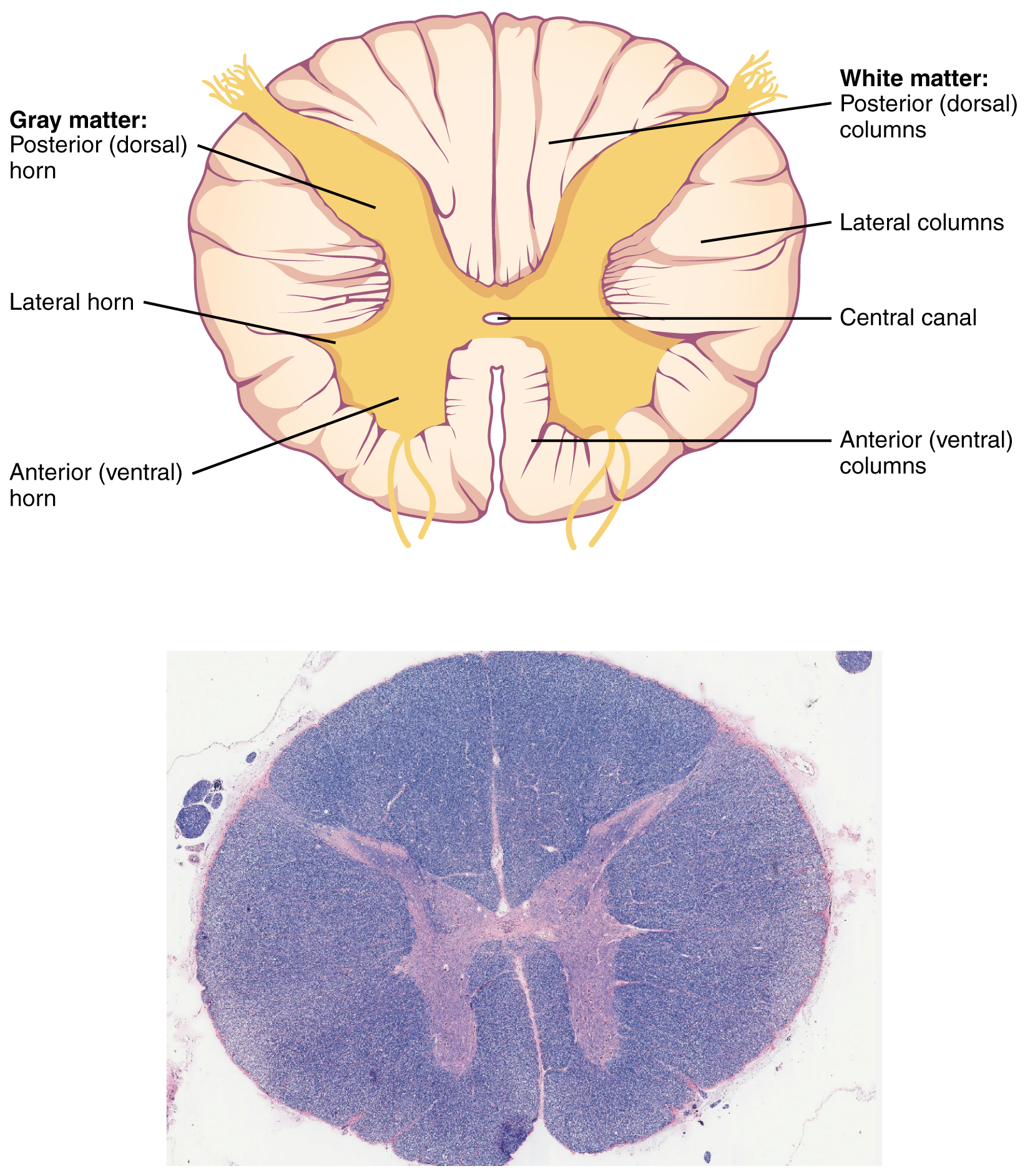
In cross-section, the spinal cord is oval or round and consists of an H– or butterfly-shaped central core of gray matter surrounded by white matter (i.e., myelinated axons traveling together over a great distance). Posterior (dorsal) roots of spinal nerves bring sensory information into the spinal cord. Ventral (anterior) roots of spinal nerves carry motor information away from the spinal cord.
Numbering Spinal Nerves and Their Roots
There are seven cervical vertebrae and eight cervical nerves. C1 nerve comes out above C1 vertebra, between the occiput of the skull and the atlas (C1 vertebra). C2 nerve comes out above C2 vertebra (axis), and so forth, until C7 nerve, which emerges from above C7 vertebra. Then we have C8 nerve, which emerges from below C7 vertebra. T1 nerve comes out below T1 vertebra, and from there down, that’s how the nerves are named: by the vertebra above.
The H-shaped gray matter is further subdivided into a posterior (dorsal) horn, which processes the sensory information brought in by the posterior root; and an anterior (ventral) horn, which contains cell bodies of α motor neurons (alpha motor neurons) controlling muscles. The axons of the α motor neurons form the anterior root of the spinal nerve, which carries motor commands going out to muscles.
At thoracic and upper lumbar levels, there is also a lateral horn (also called the intermediolateral cell column) which contains the cell bodies of the sympathetic neurons we saw in Objective 1.
The spinal cord ends in a bundle of nerves called the cauda equina (“horse’s tail”), which floats in a bag of CSF. This allows the clinician to withdraw CSF in a procedure called a lumbar puncture.
The white matter of the spinal cord is on the outside (superficial). It contains the axons of neurons that are either carrying information from the cerebral cortex of the brain to the spinal cord (called corticospinals) and from the brainstem to the spinal cord. Collectively, these are called descending tracts. There are also axons of nerve cells carrying information from the body, or from the spinal cord, to the brain. These are collectively called ascending tracts.
The white matter is further subdivided into three zones that are named pretty logically. The posterior (dorsal) horn gray matter extends to the surface and forms the “walls” of a posterior column. In the lateral white matter is the lateral column. The anterior (ventral) white matter is the anterior column.
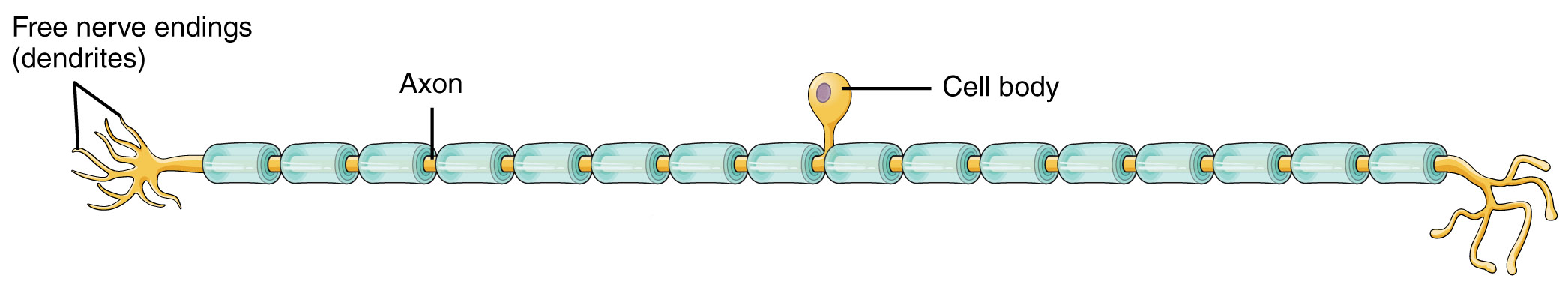
There are three basic shapes of neurons. One of these, the unipolar neuron, is found in skin sensory pathways. Modified dendrites are found in the dermis of the skin. They decide whether to create an electrical signal (action potential) within the dermis; if an action potential is created, it travels without modification along a long axon within a spinal nerve and into the posterior horn of the spinal cord directly. These axons also form the posterior roots of the spinal nerve.
Note that the previous paragraph described modified dendrites and an axon, but did not mention the cell body. Where is the cell body? It is located in a posterior root ganglion, a collection of nerve cell bodies found in the PNS. The posterior root ganglion (also called a dorsal root ganglion) is found just outside of the vertebral column and is therefore not covered by bone like the CNS structures are.
Motor information is processed and sent by α motor neurons whose axons travel out the anterior root.
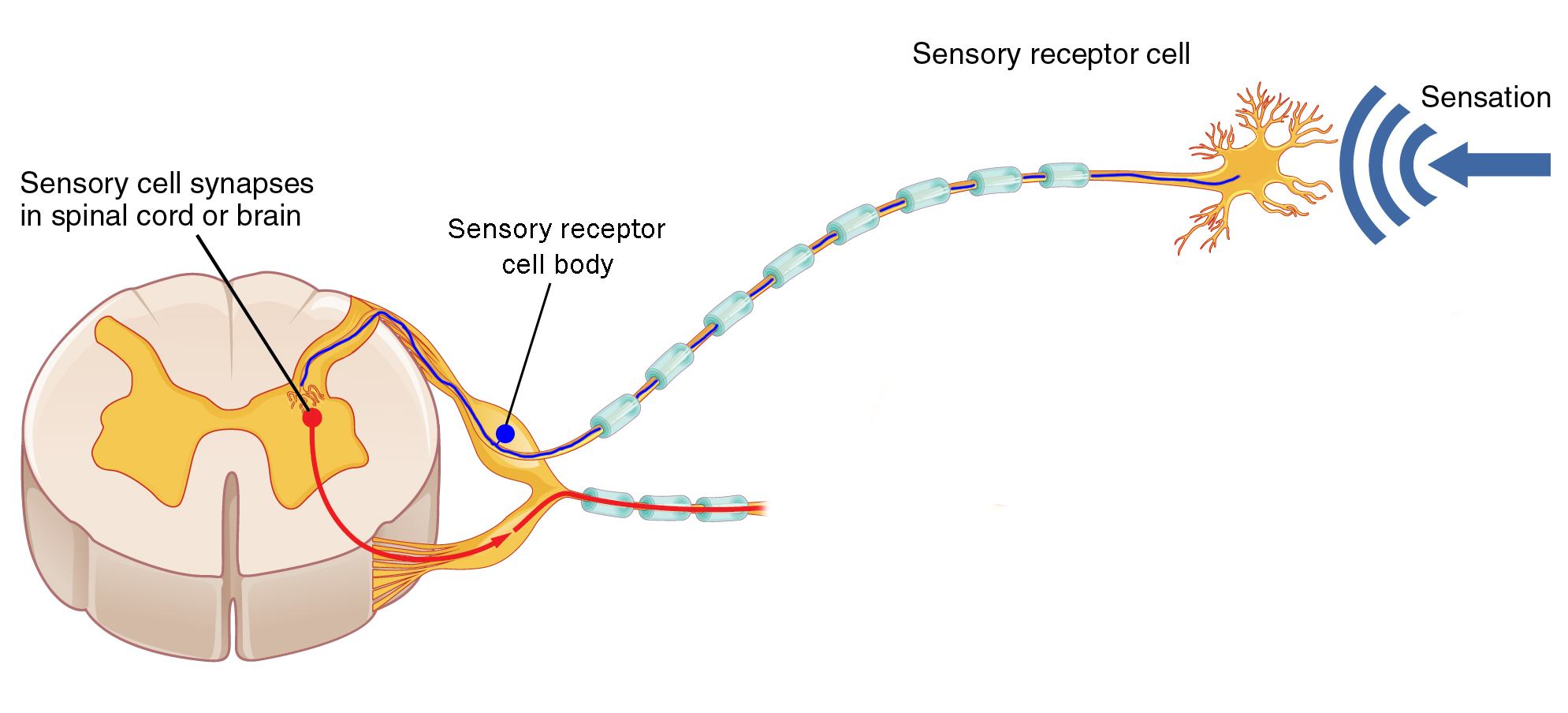
Just outside the enclosure of the vertebrae, the posterior root and anterior root join anatomically to form a spinal nerve. (Notice that the information in the spinal nerve is traveling in both directions: sensory information entering the CNS via the posterior root, and motor information leaving the CNS via the anterior root.)
As we saw in Objective 1, there is a different anatomical setup for the sympathetic nervous system. Autonomic motor neurons are found in the lateral horn from thoracic level 1 (T1) to lumbar level 2 (L2).
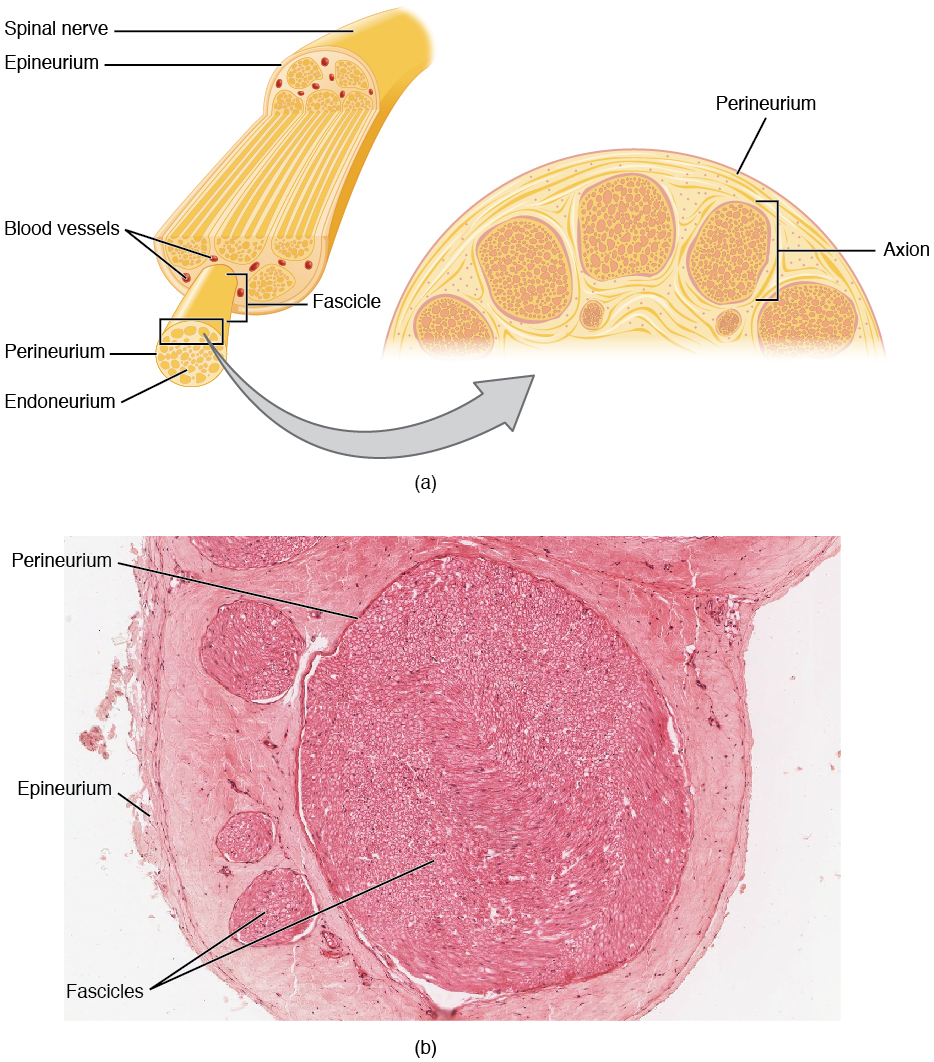
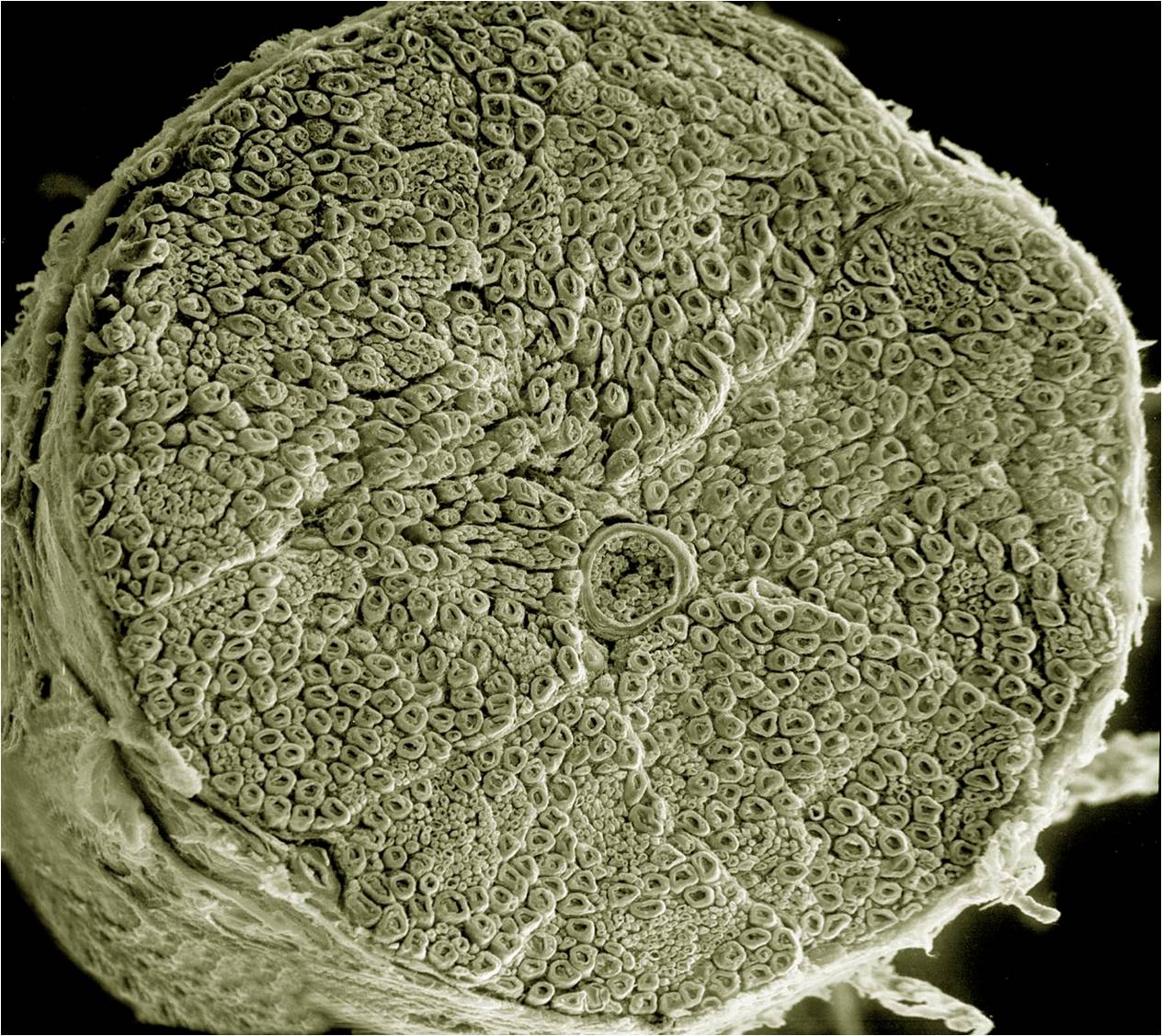
The microscopic structure of spinal nerves is shown in this diagram and scanning electron micrograph. As we’ve seen previously, spinal nerves are mixed: they contain motor neuron axons (both somatic and autonomic) as well as sensory neuron axons. Individual axons may be wrapped by Schwann cells with a myelin sheath (myelinated). Alternatively, they might be covered by a Schwann cell without being wrapped in myelin (unmyelinated).
As with muscle, there are three types of connective tissue sheath around nerve axons.
- endoneurium surrounds individual myelinated axons;
- perineurium surrounds bundles of axons, called fascicles;
- epineurium surrounds the entire nerve.
At several places in the body, spinal nerve roots and spinal nerves swap axons around so they don’t always take straight line paths. Because these structures resemble a braid, they have the Latin name for braid: plexus (plural plexi).
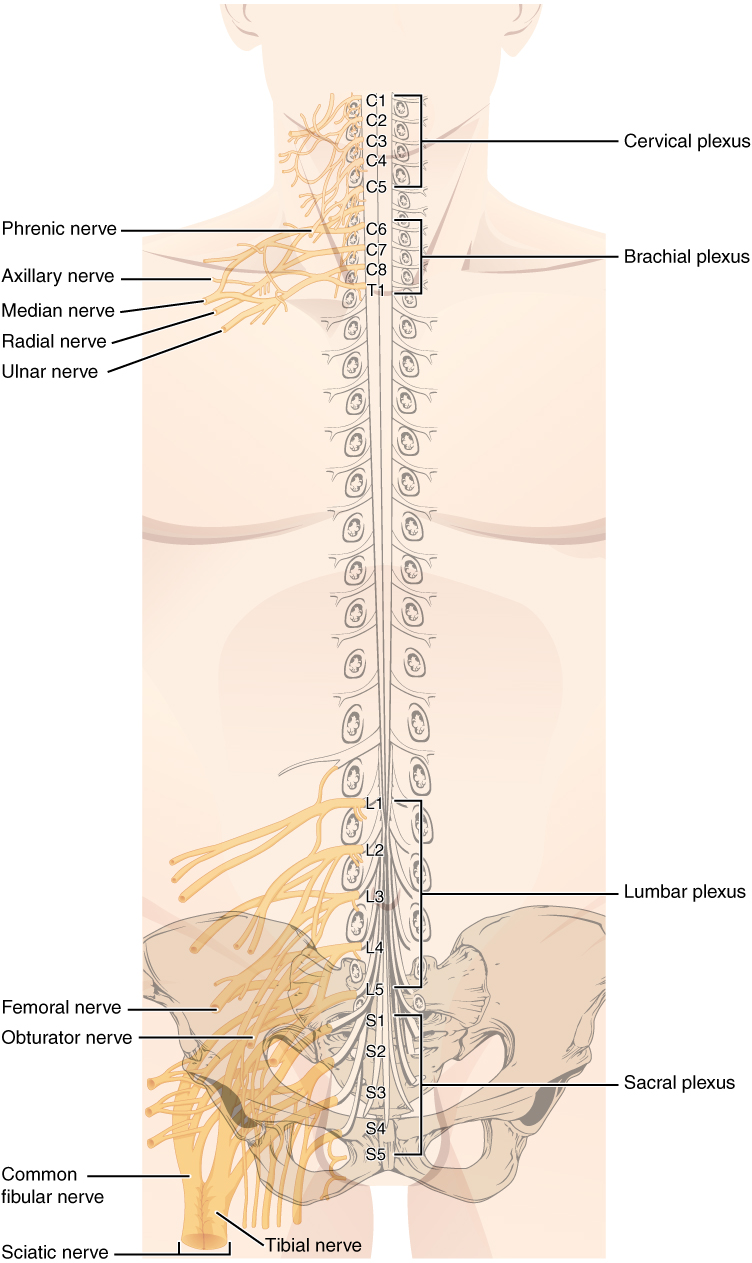 There are, in all, four of these braided nerve structures associated with the spinal cord.
There are, in all, four of these braided nerve structures associated with the spinal cord.
- The cervical plexus is in the neck. It receives nerves from the C1 through C5 spinal cord segments, and gives rise to several important nerves. The most important of these is the phrenic nerve, which innervates the diaphragm and makes breathing possible. Its contributory nerves are C3, C4 and C5, thus the mnemonic “C3, 4, 5, keep the diaphragm alive.”
- The brachial plexus receives contributions from C5-C8 and T1. Its major exiting nerves are the radial nerve (innervating the thumb and nearby structures), the median nerve (middle finger) and ulnar nerve (little finger). The nerves fight over the index and ring fingers.
- The lumbar plexus is associated with the lumbar spinal cord (roots L1–L5) and innervates the upper thigh.
- The sacral plexus receives contributions from L4–L5 and S1–S5. One huge nerve, the largest in the body, emerges from the sacral plexus: the sciatic nerve. Almost every axon innervating muscles in the leg, and almost all sensory information from the leg, is carried by the sciatic nerve. Imagine how painful inflammation or compression of this nerve would be, a condition called sciatica.
