Sturge-Weber Syndrome
Kobe Christensen
STURGE-WEBER SYNDROME
Introduction
Sturge-Weber Syndrome (SWS) is a neurocutaneous disorder caused by a mutation of the GNAQ gene located on the long arm of chromosome 9. In SWS angiomas of the leptomeninges, trigeminal nerve, and skin of the face are seen. SWS presents with seizures that generally begin as focal seizures and often progress to generalized seizures. The hallmark of SWS is a unilateral vascular lesion called the Port-Wine stain.
, via Wikimedia Commons” href=”https://commons.wikimedia.org/wiki/File:Port_wine_stains_of_an_8-year-old_female_with_Sturge-Weber_Syndrome.png”>
Babaji P, Bansal A, Choudhury GK, Nayak R, Kodangala Prabhakar A, Suratkal N, CC BY 3.0, via Wikimedia Commons
Figure 3.1 showing an 8 year old female with Sturge-Weber Syndrome with characteristic Port-Wine stain.
Signs & Symptoms
Sturge-Weber Syndrome is characterized by refractory epilepsy, neurological deficits, and physical signs of angioma.
Epilepsy
The majority of SWS patients will experience seizures within the first year of life. These seizures are described as infantile spasms and are similar to those described in children with West Syndrome. Seizures that are initially focal in nature tend to progress to generalized seizures of different types. Seizures in SWS can present as atonic, tonic, and myoclonic seizures with the incidence of absence seizures being less frequent.
EEG in Sturge-Weber Syndrome generally shows asymmetry of the hemispheres with there being an increased frequency of epileptiform activity on the ipsilateral side to the Port-Wine stain. This epileptiform typically presents as focal spike and wave discharges that can evolve to rhythmic generalized spike and wave.
Neurological Deficit
The extent of neurological deficit associated with SWS can greatly vary from patient to patient. Some patients with SWS show mild behavioral problems, while others have severe cognitive impairment. It is believed that there is a link between seizure frequency and cognitive impairment, however this has yet to be confirmed.
Another determining factor in the degree of neurological deficit is tied to the extent of brain malformation. In 85% of SWS cases, the leptomeningeal angiomas are unilateral and tend to have less severe clinical symptoms in comparison to leptomeningeal angiomas that are seen bilaterally.
Physical Signs
The hallmark of SWS is the Port-Wine stain, but patients with SWS can have several other telling clinical symptoms. Many ocular related conditions are associated with SWS including glaucoma, hemianopsia, and total vision loss. Other signs of SWS can be the development of hemiparesis ipsilateral to the Port-Wine stain, migraines, and weakness.
Treatment & Prognosis
Outcomes for SWS vary based on type of SWS, progression of bilateral leptomeningeal angiomas, severity of seizures etc. It is common for patients to continue to have refractory seizures after AED treatment and subsequent furtherment of cognitive impairment.
Medications used to treat focal seizures are the mainline treatments for epilepsy in SWS. The most common medications used are oxcarbazepine in addition to levetiracetam. Surgery is an option for patients with SWS depending on severity of seizures, location in relation to the leptomeningeal angioma, and location of seizure onset.
Key Takeaways
- Sturge-Weber Syndrome is a neurocutaneous disorder associated with the Port-Wine stain.
- Infantile spasms are common in SWS. Tuberous sclerosis caused by SWS can be the main cause for West Syndrome.
- Ocular related conditions like glaucoma and hemianopsia are related to SWS and are typically seen in the ipsilateral eye to the Port-Wine stain.
Objective 4: State the purpose of myelination and its main features.
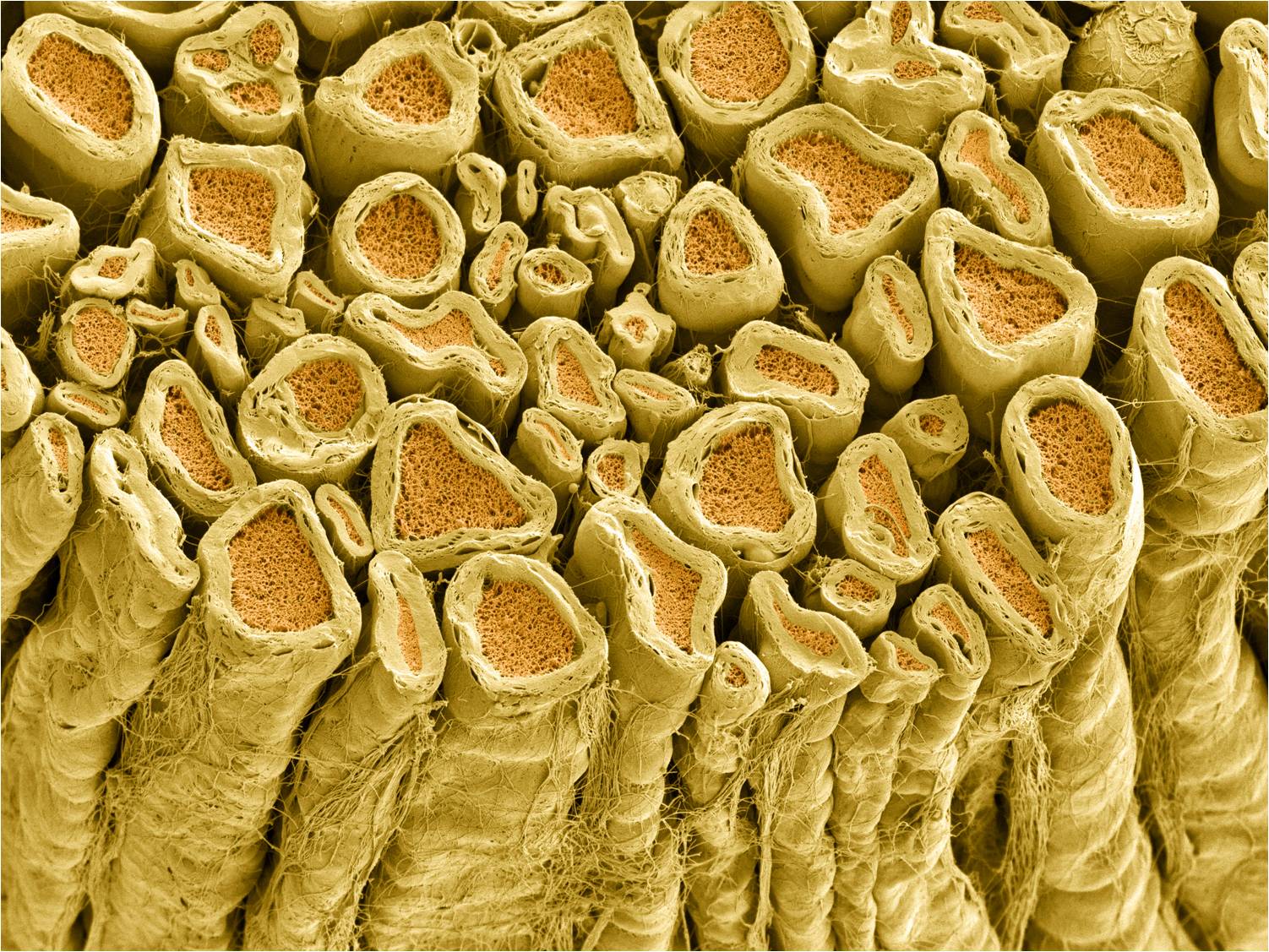 Some axons have an insulating wrapping surrounding them. We will see the function of this wrapping later, but for now, all you need to know is that myelin improves the speed and fidelity of information flow down the axon, from the cell body to the synaptic terminal. There is a difference between the myelin wrapping in the CNS, which does not support axonal regrowth in case of injury, and myelin wrapping in the PNS, which does. Both kinds of myelin are mostly (80%) lipid, but PNS myelin has specialized protein (like recognition and adhesion proteins) which supports axonal regrowth.
Some axons have an insulating wrapping surrounding them. We will see the function of this wrapping later, but for now, all you need to know is that myelin improves the speed and fidelity of information flow down the axon, from the cell body to the synaptic terminal. There is a difference between the myelin wrapping in the CNS, which does not support axonal regrowth in case of injury, and myelin wrapping in the PNS, which does. Both kinds of myelin are mostly (80%) lipid, but PNS myelin has specialized protein (like recognition and adhesion proteins) which supports axonal regrowth.
Accordingly, it’s a different cell type that makes myelin in the CNS (oligodendrocytes) and PNS (Schwann cells).
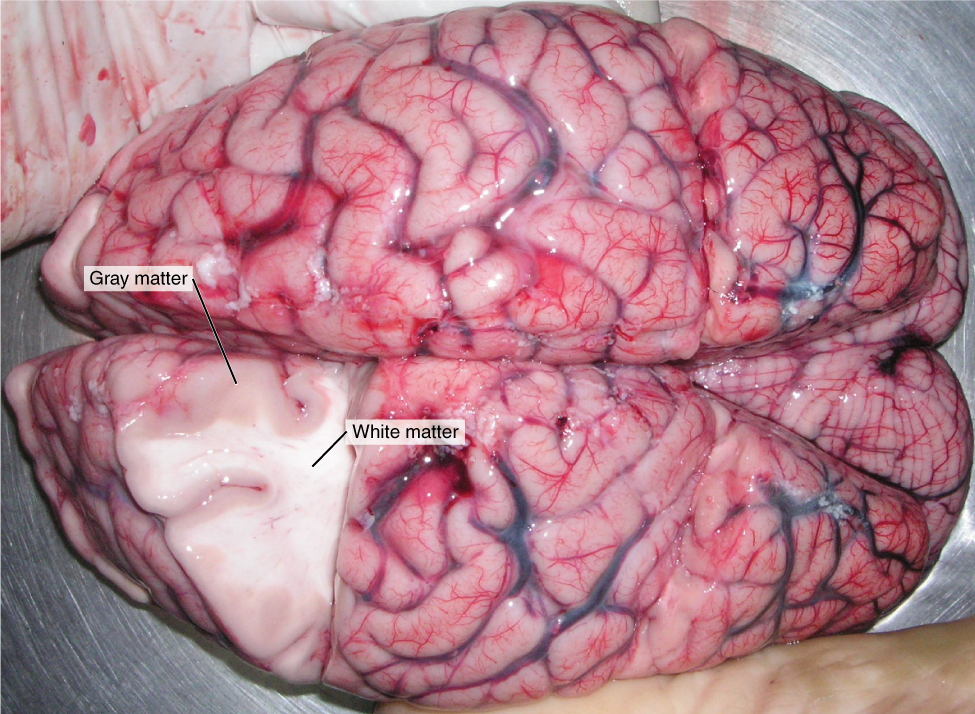 Myelin, like most lipids, appears white when you cut into it. This is the basis for so-called white matter and gray matter in the brain. (It’s really less-pink-matter and darker-pink-matter in a freshly cut or living brain, but those aren’t the terms we are given.)
Myelin, like most lipids, appears white when you cut into it. This is the basis for so-called white matter and gray matter in the brain. (It’s really less-pink-matter and darker-pink-matter in a freshly cut or living brain, but those aren’t the terms we are given.)
White matter is rich in myelinated axons and generally indicates the presence of one or more fiber tracts. Remember a tract is a bundle of neuronal axons traveling to and from the same place.
Gray matter is rich in cell bodies and dendrites. Because those are (in general) the processing parts of the neuron, most processing is done in the gray matter.
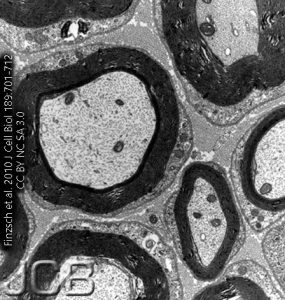
Because the phospholipid head groups and proteins found in myelin avidly bind to osmium tetroxide, in the electron microscope, myelinated axons appear to have a dense, jet-black wrapping surrounding them. This dense layer of insulating lipid will not allow many ions to cross.
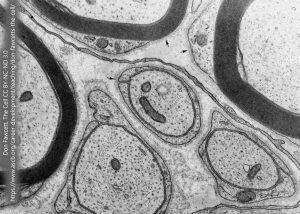
Myelinated axons carry this thick myelin sheath. Unmyelinated axons are not without protection, however. Here, we see a single Schwann cell process enrobing three unmyelinated axons in a peripheral nerve.
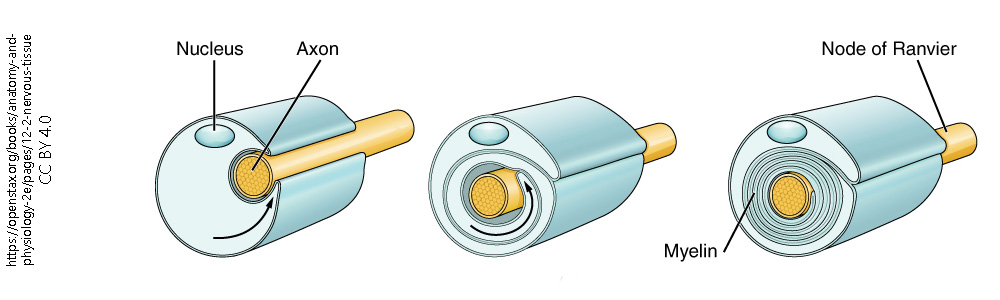
The myelin wrapping around nerves starts out as a thin extension of the oligodendrocyte or Schwann cell. In keeping with the name oligodendrocyte ("several branches cell"), there are several such thin processes that each touch a different axon. Once it touches the axon, it starts to crawl in a circular pattern over and over and over again, until several dozen layers of membrane and a thin sheet of cytoplasm surround each axon. The cytoplasm and any remaining extracellular fluid are slowly squeezed out, like toothpaste from a tube, until all that remains are lipids with the proteins which screen off negative charges and allow for cellular recognition.
