The Goldman-Hodgkin-Katz Equation
Kynzie Lalliss and Jim Hutchins
Chapter under construction. This is the first draft. If you have questions, or want to help in the writing or editing process, please contact hutchins.jim@gmail.com.
Membrane Potentials
Cellular membranes have a lipid bilayer composed of a lipid-phosphorus head and a fatty acid tail that creates both a hydrophobic and hydrophilic side to the membrane. This membrane is semi-permeable, meaning it keeps certain substances from entering the cell. Some molecules, such as water, can diffuse through the cell based on concentration gradients. Other larger molecules, like glucose and nucleotides, require channels to enter through the cell membrane.
The channels larger molecules use to enter through a cell membrane are the Na+/K+ pump, ATPase pump, ion transporters, and voltage-gated channels, which can all be found in the lipid bilayer. These regulate which ions may enter and exit the cell, which determines the concentration of ions inside the cell.
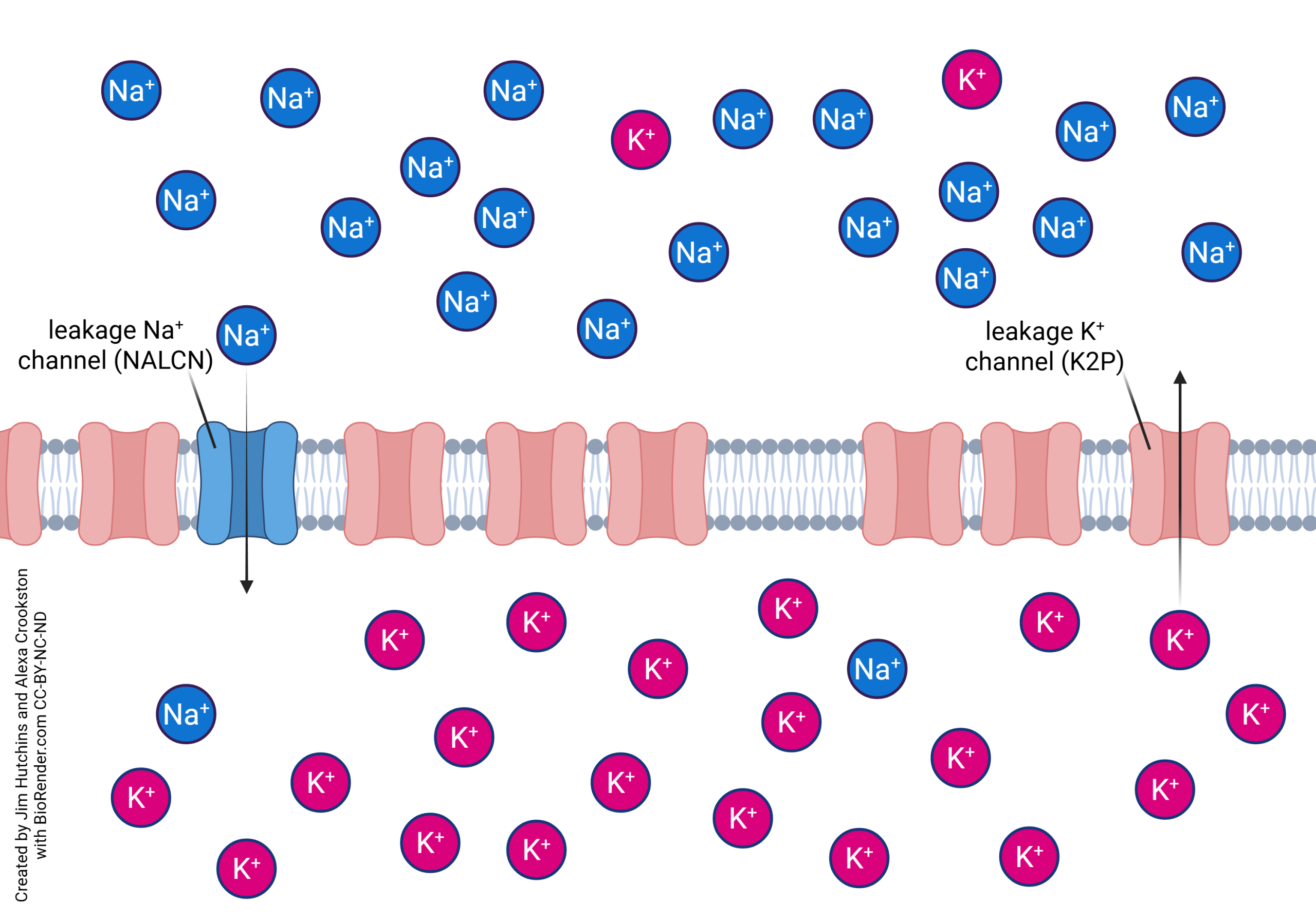
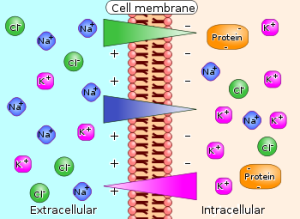
Membrane potentials are the difference in voltage, or electrical potential, between the inside and outside of a cell. The unequal concentrations of ions inside and outside of the cell is what gives the membrane an electrical charge. The most important ions that determine this difference are sodium (Na+), potassium (K+), and chloride (Cl-). Changes in these potentials elicit action potentials, which makes understanding and studying membrane potentials very important.
Determining Membrane Potential
To calculate a cell’s membrane potential, the Goldman-Hodgkin-Katz (abbreviated G-H-K) equation is used. This equation accounts for multiple ions that could potentially affect a cell’s membrane potential. These ions, as mentioned above, are sodium, potassium, and chloride (Na+, K+, Cl-).
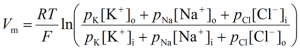
To break down this equation, Vm is the membrane potential and what the equation is measuring. R is the universal gas constant (8.314 J.K-1.mol-1), T is the temperature in Kelvin, F is Faraday’s constant (96485 C.mol-1), pion is the permeability of an ion, iono is the concentration of the ion outside the cell, and ioni is the concentration of the ion inside the cell.
History of the G-H-K Equation
The study of the membrane potential started in the 19th and early 20th centuries. In the early years of the 20th century, Bernstein hypothesized that membrane potential was due to the permeability of the membrane and the fact that potassium was higher inside the cell and lower on the outside of the cell. Walther Nerst also majorly contributed to the study of membrane potential by developing the Nernst equation to solve for the equilibrium potential of one specific ion. Goldman, Hodgkin, and Katz built upon the Nernst equation and developed the G-H-K equation to solve for membrane potential that accounts for any ion that ma permeate the membrane and affect its potential.
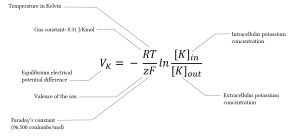
The only difference between the Nernst equation and the Goldman-Hodgkin-Katz equation is that the Nernst equation only solves for one ion’s equilibrium, while the G-H-K sums up the equilibriums of sodium, potassium, and chloride to find a cell’s overall membrane potential.
Media Attributions
- Resting Neuronal Membrane Ion Distribution and Leakage Channels © Jim Hutchins and Alexa Crookston is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- Membrane Picture
- Goldman Equation
- Nernst equation

