The Endomembrane System, Exocytosis, and Endocytosis in Neurons
Jim Hutchins
Chapter under construction. This is the first draft. If you have questions, or want to help in the writing or editing process, please contact hutchins.jim@gmail.com.
Exocytosis in Neurons
Exocytosis in neurons takes on a critical role because most neurons communicate with each other using chemical extracellular signals called neurotransmitters.
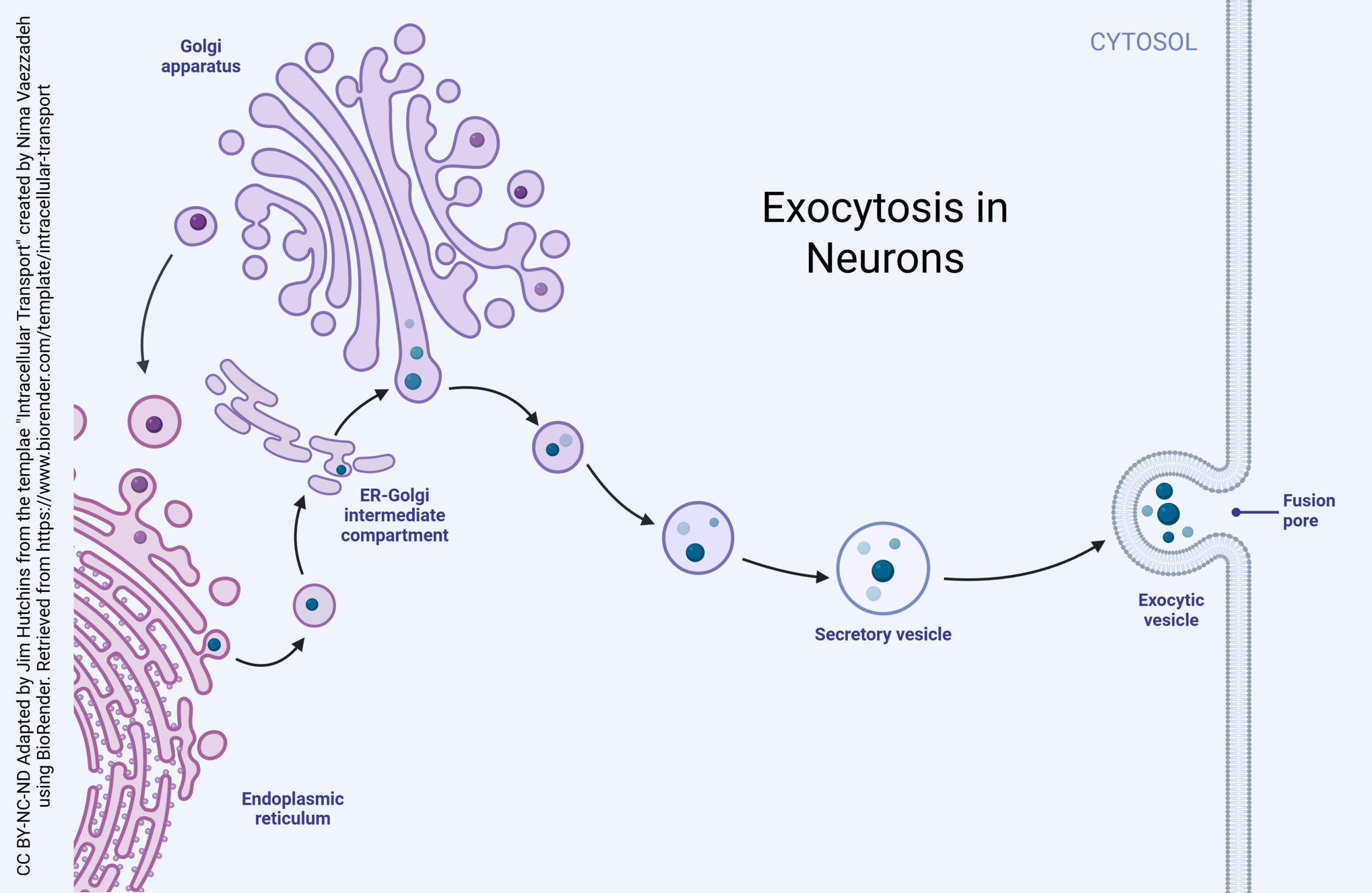
Proteins are made in a macromolecular machine called a ribosome. Ribosomes stitch together amino acids, the monomers of proteins, using the messenger RNA code as a template. The details of this process are explained elsewhere.
Proteins for export or proteins that will be expressed on the cell surface (such as pumps, channels, or neurotransmitter receptors) are made in the rough endoplasmic reticulum (rough ER), an organelle consisting of ribosomes attached to the endomembrane system. Protein modification then begins.
First, proteins travel into the ER-Golgi intermediate complex (ERGIC). Vesicles containing proteins bud off of the rough endoplasmic reticulum and take on an outer layer (coat) of coat protein complex II (COPII). These vesicles then fuse to form the cis face of the Golgi.
(The Golgi apparatus, also called the Golgi complex, forms at the cis face and breaks apart at the trans face. These faces are named for their relationship to the ER, with the cis face closer to the ER and the trans face further away.)
When there is a need to move proteins in the opposite direction, from the cis face of the Golgi to the rough ER, they are coated with coat protein complex Ia.
-
Carbohydrate Synthesis Stage: A transition occurs when a cis cisterna loses the ability to receive COPII vesicles while acquiring the ability to receive glycosylation enzymes and sugar nucleotide transporters in COPIb vesicles. These processes convert the cis cisterna into a medial cisterna. Medial cisternae mature into trans cisternae. Both medial and trans cisternae are involved in carbohydrate synthesis, with early-acting enzymes concentrated in medial cisternae and late-acting enzymes in trans cisternae. Cisternae at the carbohydrate synthesis stage exchange material with one another via COPIb vesicles.
-
Carrier Formation Stage: A transition occurs when a trans cisterna loses the ability to receive COPIb vesicles, and subsequently loses the ability to produce COPIb vesicles while acquiring the ability to produce clathrin-coated vesicles. The timing of secretory vesicle formation and the ultimate fate of carrier formation cisternae are still poorly understood. ER membranes attached to trans and/or TGN cisternae probably function in direct lipid transfer between the organelles (Hanada et al. 2009). This diagram depicts the mammalian TGN as the last cisterna in the stack.
Regulated and Constitutive Secretion in Neurons
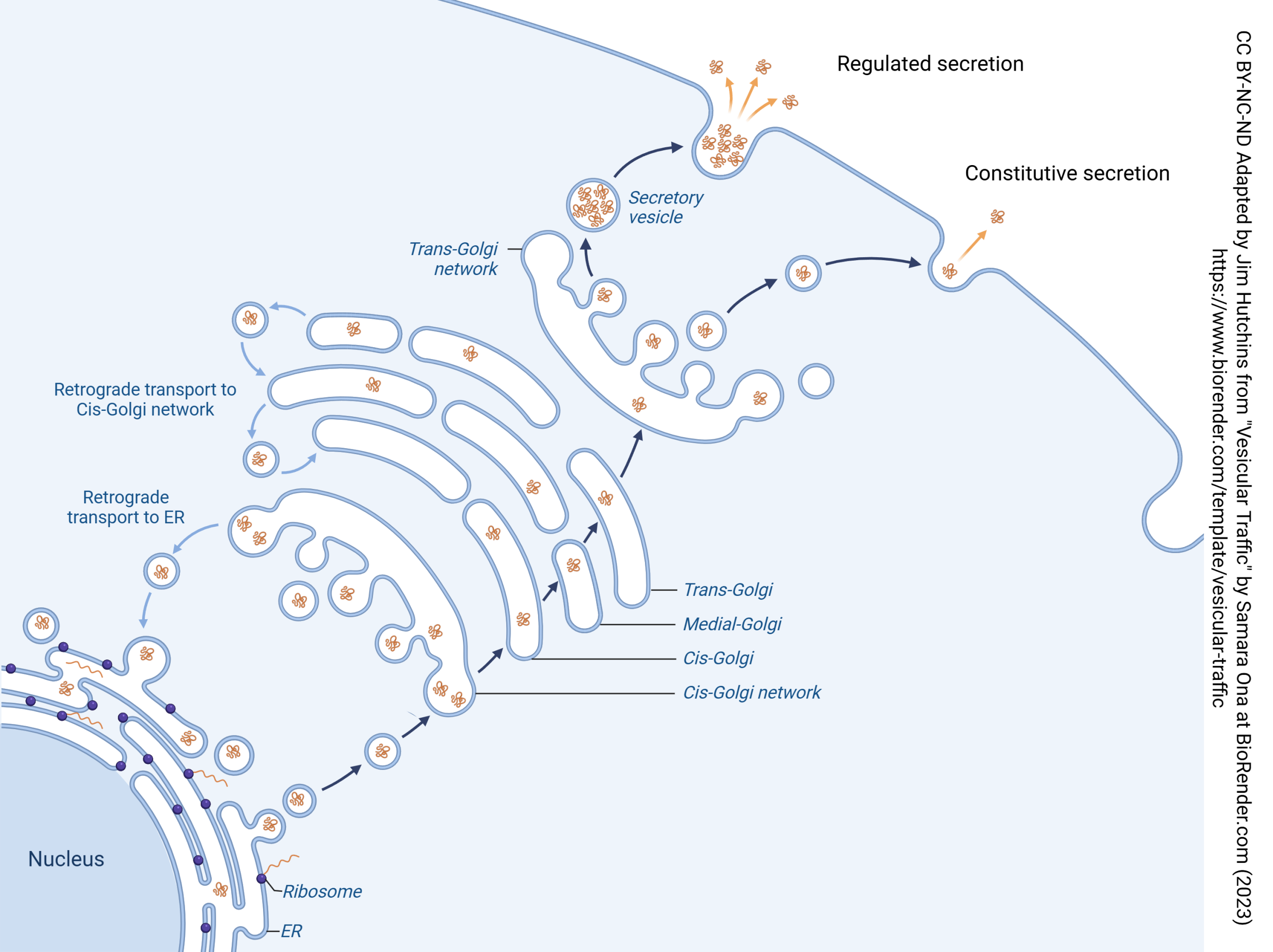
Vesicular release of neurotransmitter is an extreme example of regulated secretion: secretion that only occurs when specialized conditions exist. In the case of synaptic release, the specialized condition is entry of Ca2+. Neurons, and other cells, may regulate exocytosis through other signaling pathways.
Not all secretion is regulated. Neurons, and other cells, may constantly make proteins and release them 24/7/365.2422. This type of secretion is called constitutive secretion. The word “constitutive” is used by biologists to mean “occurring constantly”.
Neurotransmitter Release Is a Specialized Kind of Exocytosis
 All cells are capable of secreting chemical substances to regulate the cells around them. The general name for this type of secretion is paracrine secretion to distinguish it from endocrine secretion, the release of chemical substances into the bloodstream to act on distant organs. Because neurotransmitters generally act over short distances (less than 1 μm) and for short periods of time, neurotransmission is a highly specialized and regulated form of paracrine secretion.
All cells are capable of secreting chemical substances to regulate the cells around them. The general name for this type of secretion is paracrine secretion to distinguish it from endocrine secretion, the release of chemical substances into the bloodstream to act on distant organs. Because neurotransmitters generally act over short distances (less than 1 μm) and for short periods of time, neurotransmission is a highly specialized and regulated form of paracrine secretion.
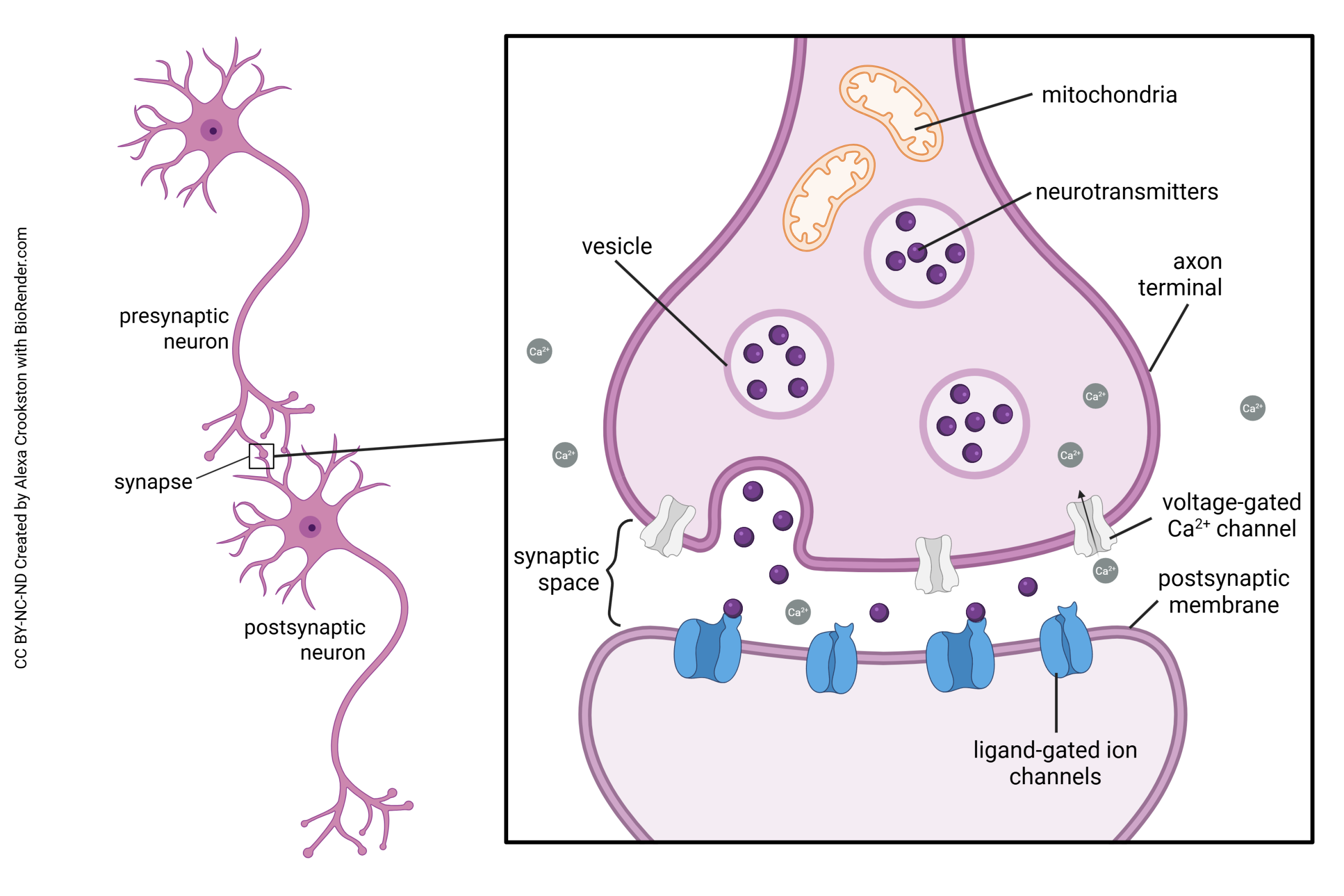
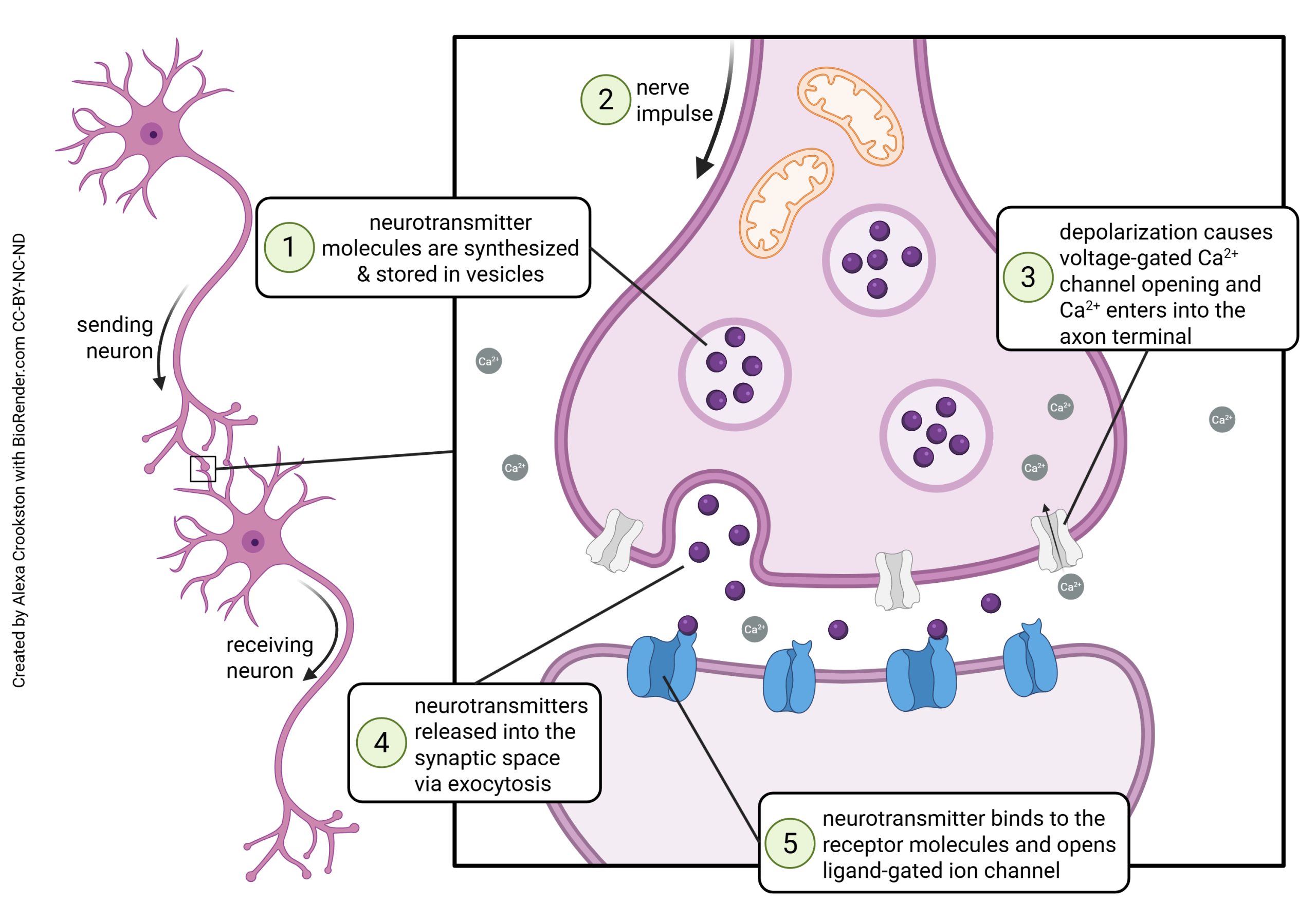 Neurotransmission occurs at a structure called the synapse. The word “synapse” (from the Greek word synaptein, “to clasp”) was coined by Charles Scott Sherrington in 1897 to describe a theoretical concept, long before anyone actually saw a synapse as drawn above.
Neurotransmission occurs at a structure called the synapse. The word “synapse” (from the Greek word synaptein, “to clasp”) was coined by Charles Scott Sherrington in 1897 to describe a theoretical concept, long before anyone actually saw a synapse as drawn above.
Some of the steps in vesicle fusion and neurotransmitter release at the synapse will be discussed later. Now, we are focusing on the mechanisms of neurotransmitter exocytosis at the synapse.
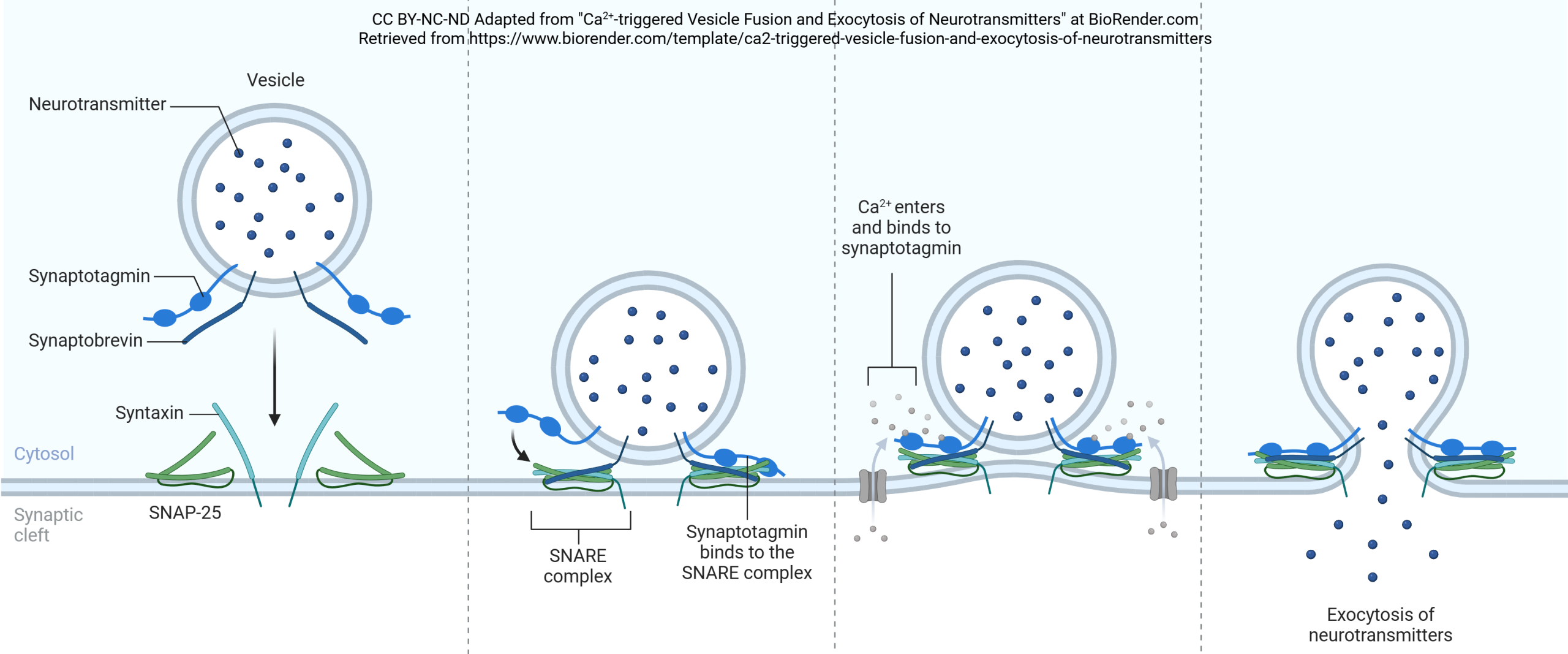 Synaptic vesicles are coated with a variety of proteins including synaptotagmin and synaptobrevin. Synaptobrevin is also called VAMP. The deadliest substance known to mankind, botulinum toxin, works by cleaving synaptobrevin and thereby blocking neurotransmitter release. (One gram of botulinum toxin, properly dispersed, would kill 1,000,000 people; gram for gram, it’s 1000X more deadly than plutonium.)
Synaptic vesicles are coated with a variety of proteins including synaptotagmin and synaptobrevin. Synaptobrevin is also called VAMP. The deadliest substance known to mankind, botulinum toxin, works by cleaving synaptobrevin and thereby blocking neurotransmitter release. (One gram of botulinum toxin, properly dispersed, would kill 1,000,000 people; gram for gram, it’s 1000X more deadly than plutonium.)
Synaptotagmin is sensitive to calcium ion (Ca2+) levels in the neuronal cytoplasm. When Ca2+ levels rise, synaptotagmin and synaptobrevin attach to two proteins that are found on the inner surface of the cell membrane at the synapse, syntaxin and SNAP-25. Together, these proteins form a SNARE complex. Ca2+ entry causes the SNARE complex to collapse, drawing the vesicle close to the presynaptic membrane. Fusion between the vesicle membrane and presynaptic membrane occurs, and neurotransmitter is released through exocytosis.
Endocytosis in Neurons
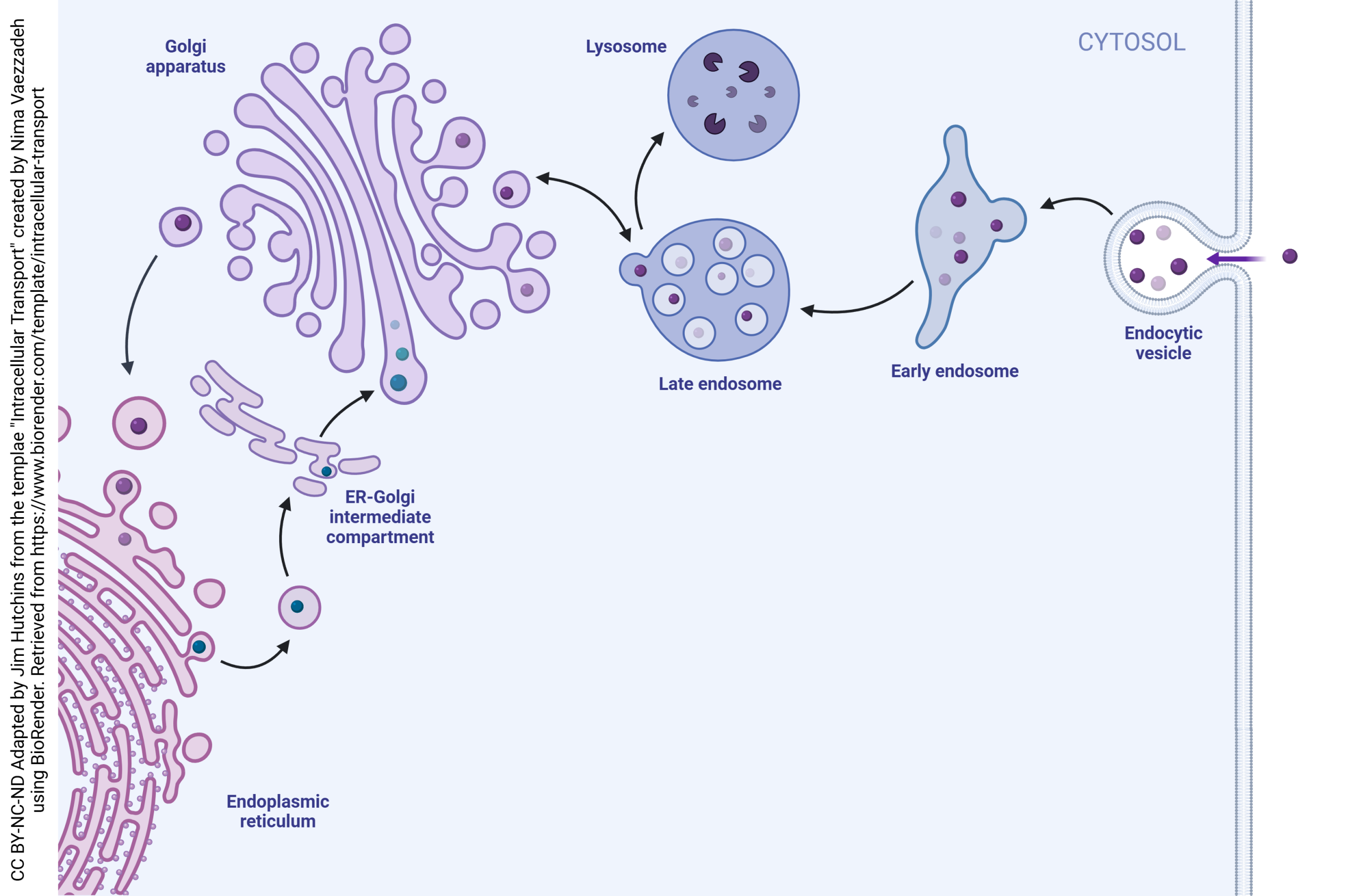
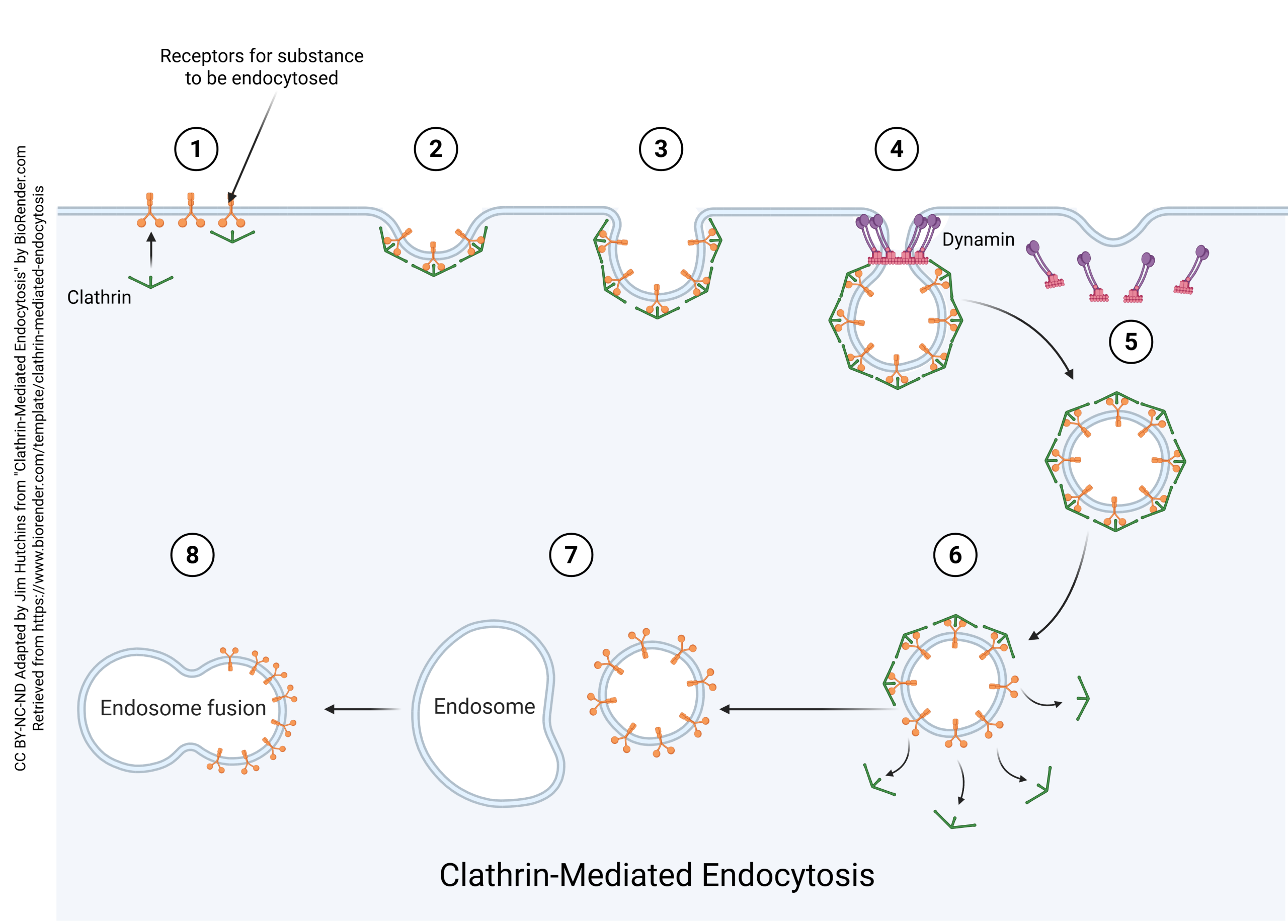
Neurons also take substances back into the cell and recycle vesicles. This process is called endocytosis. If there were no endocytosis, the surface area of the neuronal cell membrane would consistently increase in tiny increments until the cell became significantly larger. Also note that the vesicle membrane is made up of the same components as the cell membrane, which means that vesicular release and endocytosis do not change the markers found on the surface of the cell.
When substances are to be taken up by a neuron, they form an endocytic vesicle. Several endocytic vesicles fuse to form the early endosome. The early endosome has the same contents as the original endocytic vesicles.
Then, the late endosome forms through interaction with the trans face of the Golgi. Substances move back and forth between the late endosome and the Golgi. Eventually, the late endosome contents fuse with the lysosome and are destroyed. The constituent lipids, carbohydrates, and amino acids can then be recycled into other substances by the neuron.
Receptor-Mediated Endocytosis (Clathrin-Mediated Endocytosis)