The Cyclic Adenosine Monophosphate Second Messenger System
Jim Hutchins
Chapter under construction. This is the first draft. If you have questions, or want to help in the writing or editing process, please contact hutchins.jim@gmail.com.
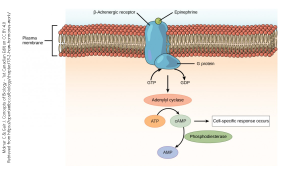
The most common outcome when neurotransmitter binds to a G protein-coupled receptor (GPCR) is the creation of a key intracellular signaling molecule called cyclic adenosine monophosphate (cyclic AMP or cAMP).
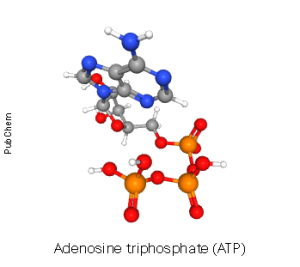 The enzyme that converts adenosine triphosphate (ATP) to cyclic adenosine monophosphate is called adenylate cyclase or sometimes adenylyl cyclase. Remember that “–ase” is the word ending we use for enzymes and forming a “cycle”, or ring, is what this enzyme does to adenosine. Adenosine is the nucleic acid base adenine with a ribose sugar added. Adenosine triphosphate is adenine + ribose + three phosphates.
The enzyme that converts adenosine triphosphate (ATP) to cyclic adenosine monophosphate is called adenylate cyclase or sometimes adenylyl cyclase. Remember that “–ase” is the word ending we use for enzymes and forming a “cycle”, or ring, is what this enzyme does to adenosine. Adenosine is the nucleic acid base adenine with a ribose sugar added. Adenosine triphosphate is adenine + ribose + three phosphates.
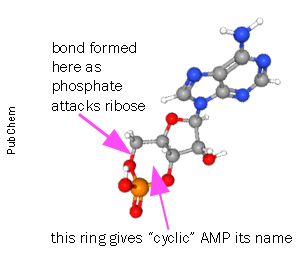
The enzyme adenylate cyclase (AC) removes two of the three phosphates in ATP and the remaining phosphate attacks the ribose ring structure to form a new ring, the cycle that gives cyclic AMP its name. The enzyme that breaks this linkage, rendering cAMP into its inactive form (adenosine monophosphate, AMP) is called phosphodiesterase after the chemical name for the type of bond that it breaks (a phosphodiester bond).
The term of art for cAMP is a second messenger. The extracellular signaling molecule (generally, a neurotransmitter, hormone, or drug) which brought the message to the receptor is the first messenger, although you will almost never hear this term used. The first messenger causes the shape of the G protein-coupled receptor to change. When the shape of the GPCR changes, it activates the associated G protein, which consists of alpha (α), beta (β), and gamma (γ) subunits, to exchange its guanosine diphosphate (GDP), which is bound to the inactive form, with guanosine triphosphate (GTP), which is bound to the active form. As it activates, the shape of the G protein complex changes and it no longer likes to hang out with the receptor. It releases from the GPCR and breaks apart. In general, the α subunit of the G protein has one function, while the βγ subunits together carry out another function. See the chapter on metabotropic receptors for a review of this topic.
It is the α subunit which generally interacts with the enzyme adenylate cyclase.
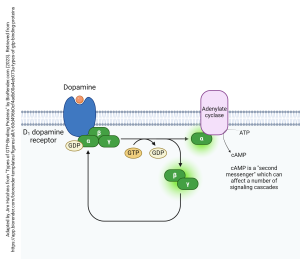 An example of how this system works is shown here, with the neurotransmitter dopamine binding to its GPCR (in this case, the specific receptor bound is called the D1 dopamine receptor; there are five types, numbered D1 through D5). When dopamine binds, the G protein complex (αβγ) exchanges GDP for GTP. It releases from the D1 dopamine receptor. All parts of the G protein carry lipid tails which remain in the membrane, constraining these protein subunits to remain just inside the cell membrane on the cytoplasmic side. Upon release, the G protein splits a phosphate group off GTP and creates GDP. This causes the G protein to split apart into an α subunit which bangs into adenylate cyclase and activates it. The activated adenylate cyclase turns ATP into cAMP, as shown.
An example of how this system works is shown here, with the neurotransmitter dopamine binding to its GPCR (in this case, the specific receptor bound is called the D1 dopamine receptor; there are five types, numbered D1 through D5). When dopamine binds, the G protein complex (αβγ) exchanges GDP for GTP. It releases from the D1 dopamine receptor. All parts of the G protein carry lipid tails which remain in the membrane, constraining these protein subunits to remain just inside the cell membrane on the cytoplasmic side. Upon release, the G protein splits a phosphate group off GTP and creates GDP. This causes the G protein to split apart into an α subunit which bangs into adenylate cyclase and activates it. The activated adenylate cyclase turns ATP into cAMP, as shown.
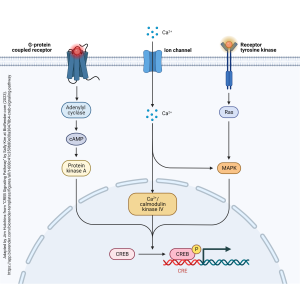
The cAMP second messenger is one of at least three ways that gene expression can be altered. The cAMP second messenger pathway which alters gene expression is shown at left in this diagram. Cyclic AMP causes the phosphorylation of an enzyme called protein kinase A. Protein kinases are enzymes which add a phosphate group onto key regulatory proteins. In this case, protein kinase A transfers a phosphate group onto a transcription factor called CREB. CREB is covered in the Introduction to Neuroscience book as one of the “molecules of memory”. Phosphorylated CREB binds to the CREB-responsive element (CRE) which is part of key gene sequences, and turns those genes “on” or “off”.
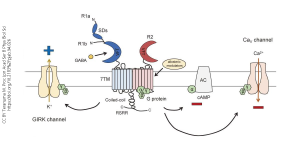
The GABAB receptor is one of the main ways the neurotransmitter γ-aminobutyric acid (GABA) exerts an inhibitory, or hyperpolarizing, effect. (Recall the GABAA receptor is an ionotropic receptor which allows Cl– into the cell, also causing it to hyperpolarize.) In the case of the GABAB receptor, the binding of GABA causes the dissociation of the G protein with the α subunit inhibiting the enzyme adenylate cyclase (AC).
Wait! Earlier we said that the α subunit excites the enzyme adenylate cyclase. Which is right? Well, both. There are three types of α subunit. The one associated with the D1 dopamine receptor stimulates adenylate cyclase and is therefore called an αs subunit. The one associated with the GABAB receptor inhibits adenylate cyclase and is therefore called an αi subunit. (There is a third one, called αq, which will be discussed later.)
Although we are currently focused on cAMP levels, you can see in the diagram above that the βγ subunits also evoke an inhibitory response, by restricting the opening of Ca2+ channels and by opening the G protein-gated inwardly rectifying K+ channels, so-called GIRK channels. “Inward rectification” refers to the detailed electrical properties of this channel. What you need to know is that when GIRK channels open, or open longer, after being phosphorylated by the action of the βγ subunits, K+ rushes out of the cell and a cell which loses positive ions becomes electrically more negative inside — it hyperpolarizes. (You could also figure this out from the Goldman-Hodgkin-Katz equation: as the K+ conductance increases, the neuron reaches closer to the K+ equilibrium potential, which is more negative than rest.)
GABA, acting through GABAB receptors, inhibits neuronal firing by decreasing cAMP levels, by blocking the entry of Ca2+, and by hyperpolarizing the neuron when GIRK channels are phosphorylated and therefore active.
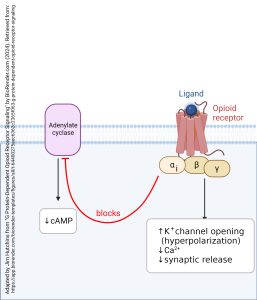
When the αi subunit is part of a G protein complex, the entire complex is called Gi.
Another example of the action of a Gi protein is the G protein associated with the opioid receptor. These are the receptors which respond to the endogenous neurotransmitter endorphin and its drug analogs such as morphine, opium, heroin, oxycodone, or fentanyl (among many others). The name “endorphin” is a shortening of “endogenous morphine”. When the ligand, either the naturally occurring transmitter or a drug, binds to the opioid receptor, the αi subunit inhibits adenylate cyclase, reducing the amount of cAMP made; the βγ subunits (like those associated with the GABAB receptor) open the GIRK channel and close Ca2+ channels, reducing synaptic release of neurotransmitter.
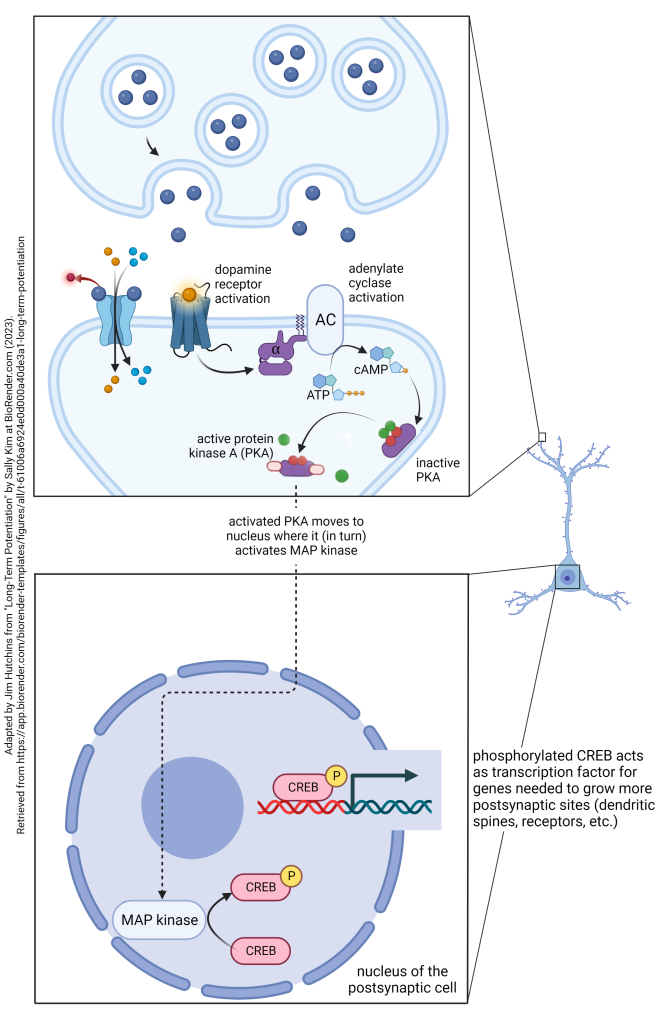
As explained in the Introduction to Neuroscience chapter on Early and Late Long-Term Potentiation, dopamine plays a key role in changing gene expression which supports long-term potentiation (i.e. strengthening synapses) lasting for years, decades, or an entire lifetime. We are now positioned to see how dopamine acts to reinforce permanent memory formation.
Many people learn that dopamine is a “pleasure neurotransmitter” or “reward neurotransmitter“. That’s sorta correct, but not truly accurate. It would be better to say dopamine is a “reinforcement neurotransmitter”. That is, it connects the relatively short-term process of long-term potentiation to the lifelong process of memory formation by turning on genes which create and strengthen synaptic contacts.
This is done through the dopamine receptor’s G protein. D1 and D5 dopamine receptors are associated with a Gs protein that contains an αs subunit. When the αs subunit interacts with adenylate cyclase, it increases the production of cAMP. This, in turn, activates a key source of protein phosphorylation, protein kinase A (PKA). PKA then moves to the nucleus, where it adds a phosphate group to the mitogen-associated protein (MAP) kinase. The activated, phosphorylated MAP kinase adds a phosphate group to CREB. The phosphorylated CREB then acts as a transcription factor at the CRE region of genes including those that make new synapses, those that maintain synapses by inserting glutamate receptors in the postsynaptic membrane, and a host of other functions. In this way, the existing synaptic contacts are strengthened, and new synaptic contacts are made. Now a memory has become a permanent part of your life. This process is accelerated by an emotional salience to the memory; emotions cause more dopamine release from the nucleus accumbens septi and other brain areas. We have all had the experience of what psychologists call a “flashbulb memory” — those that have such a strong emotional component that they are immediately turned into permanent brain circuitry changes. Dopamine plays a key role in those, and not all of those are pleasant.
Media Attributions
- Neurotransmitter GPCR transduction © Molnar, Charles and Gair, Jane is licensed under a CC BY-SA (Attribution ShareAlike) license
- ATP ball and stick © PubChem adapted by Jim Hutchins is licensed under a Public Domain license
- cAMP ball and stick © PubChem adapted by Jim Hutchins is licensed under a Public Domain license
- Dopamine D1 receptor © BioRender adapted by Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- CREB Signaling Pathway in Neurons © BioRender adapted by Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- GABA b receptor © Miho Terunuma is licensed under a All Rights Reserved license
- Opioid receptors are an example of inhibitory G protein signaling © BioRender adapted by Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- Long-Term Potentiation step 4 © Sally Kim adapted by Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license

