Synaptic Pruning, Synaptic Stabilization, and Autism
Aliyah Grijalva and Jim Hutchins
Chapter under construction. This is the first draft. If you have questions, or want to help in the writing or editing process, please contact hutchins.jim@gmail.com.
The Importance of Being Pruned
All areas of the mammalian brain overproduce synapses; those synapse numbers must then be reduced through a process described in the previous chapter, synaptic pruning. An imbalance in the normal amount of synaptic pruning may be (at least in part) responsible for the autistic phenotype. Researchers have speculated that having more synapses is consistent with the observation that autistic individuals often have over-sensitivity issues. Too many inputs may be the brain is dealing with unnecessary noise.
Autism and the mTOR Pathway
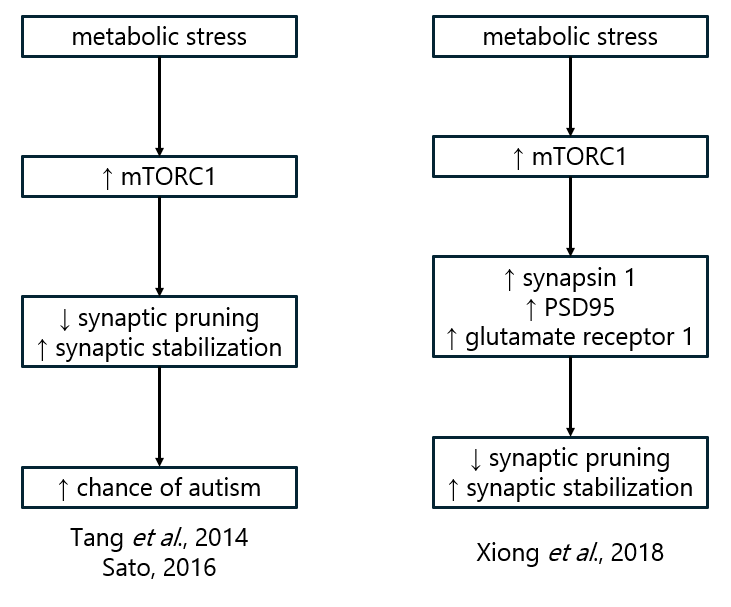 The mechanistic (or mammalian) target of rapamycin (mTOR), a kinase which promotes the survival of cells and parts of cells (like dendritic spines), is strongly associated with autism-like wiring of the brain. The original paper linking mTOR and autism was by Tang et al., published in 2014. An increase in mTOR causes an increase in neuronal survival, particularly of dendritic spines in neurons, and this in turn causes an autism-like phenotype. The idea is that mTOR activation allows survival of too many dendritic spines, and the neuron receives more input than normal that it has to sort through and integrate.
The mechanistic (or mammalian) target of rapamycin (mTOR), a kinase which promotes the survival of cells and parts of cells (like dendritic spines), is strongly associated with autism-like wiring of the brain. The original paper linking mTOR and autism was by Tang et al., published in 2014. An increase in mTOR causes an increase in neuronal survival, particularly of dendritic spines in neurons, and this in turn causes an autism-like phenotype. The idea is that mTOR activation allows survival of too many dendritic spines, and the neuron receives more input than normal that it has to sort through and integrate.
Complement C4
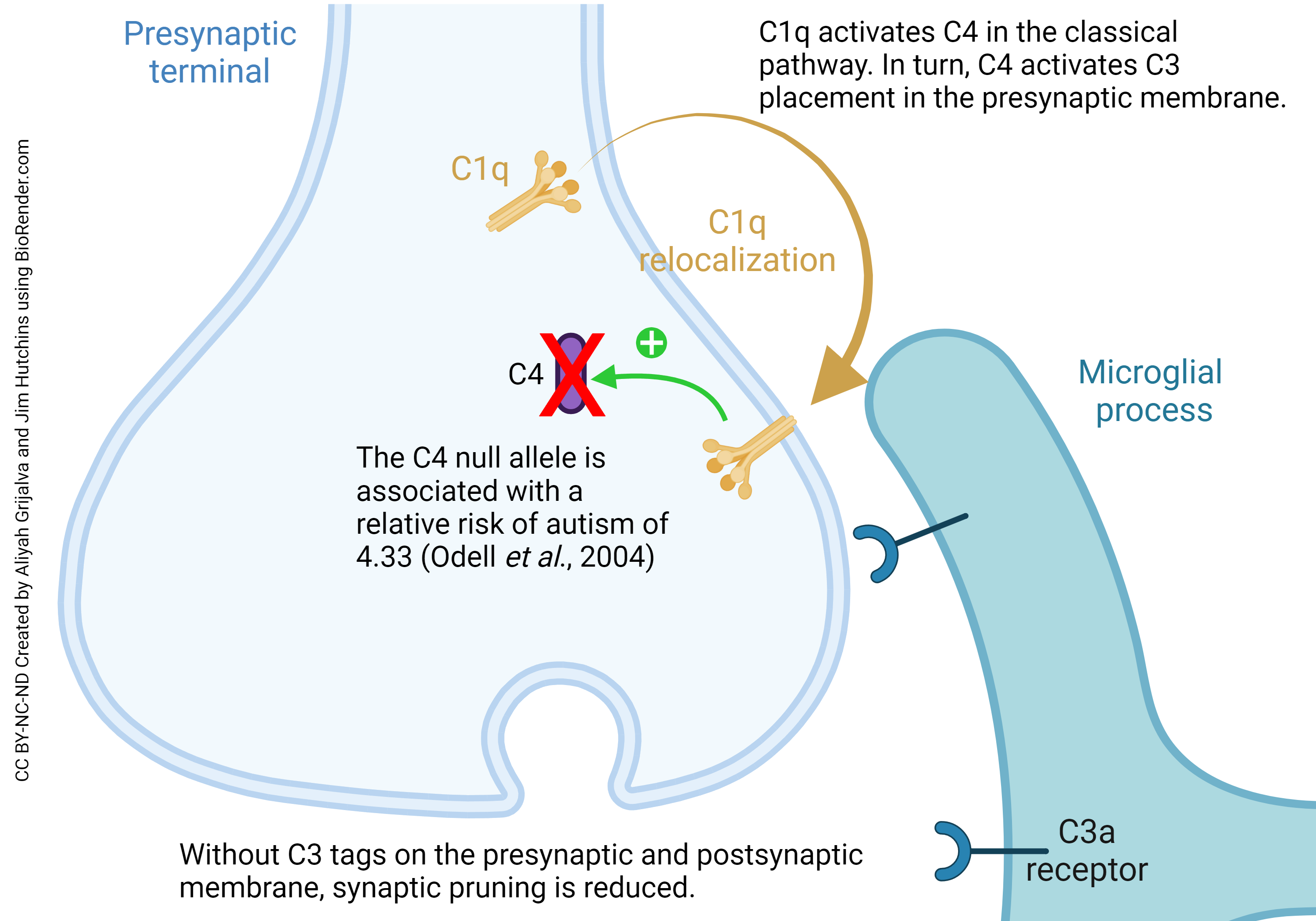
Low levels of, or a mutation in, complement C4 also can mimic the autism phenotype. C4 tags synapses and targets them for pruning (“complement mediated synaptic pruning”). It marks which ones should be eliminated by microglia through synapse engulfment, which can be phagocytosis or trogocytosis. Low levels of C4 are correlated with autism. When C4 is low, there is less tagging of synapses for destruction and thus less synaptic pruning overall. Synaptic pruning is most intense during early development but in humans can continue into late childhood and early adolescence. Excessive synaptic pruning may even be involved in neurodegenerative diseases of aging, such as Alzheimer disease.
Rho Family GTPases
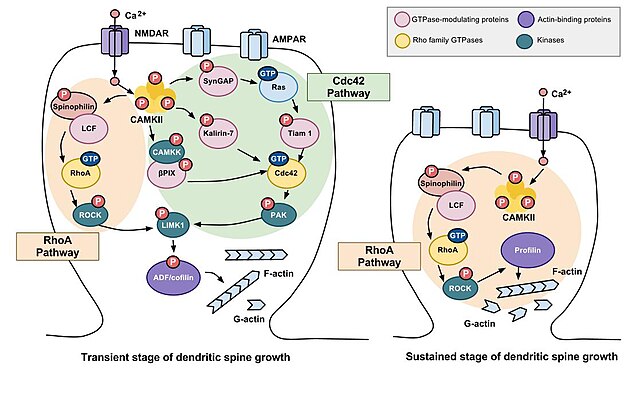
A number of intracellular signaling pathways have been implicated in synaptic pruning defects like those seen in autism. One that seems to be strongly associated with autism is the Rho family of GTPases, an intracellular signaling mechanism that results in the stabilization of actin filaments. The stabilized filaments then form a cytoskeletal core that supports the structure of the spine and helps protect it from pruning.
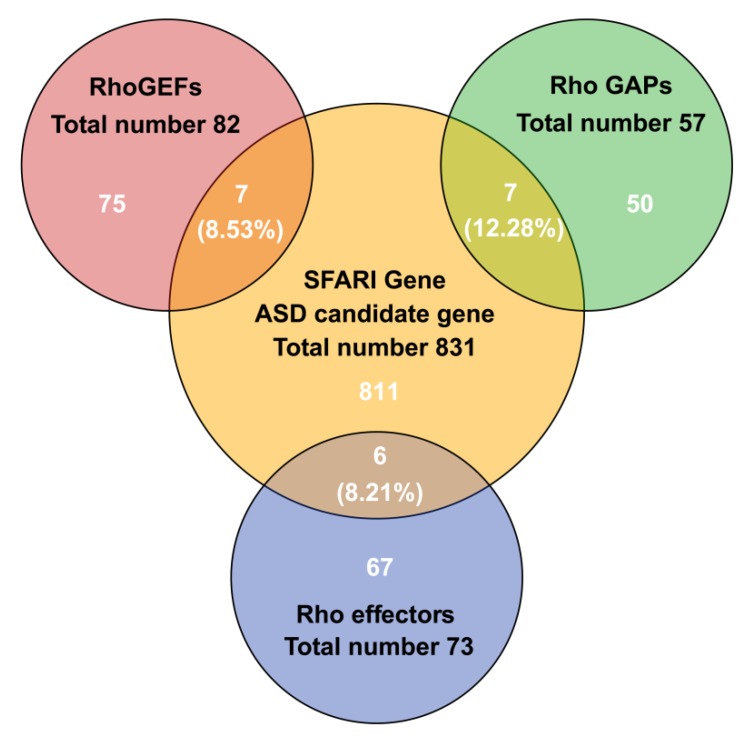
Of the genes listed by in the Simons Foundation Autism Research Initiative (SFARI) database, 2.4% belong to the Rho GTPase family. This observation fits well with the central role of the actin cytoskeleton in supporting the structure of dendritic spines and other synapse-associated structures.
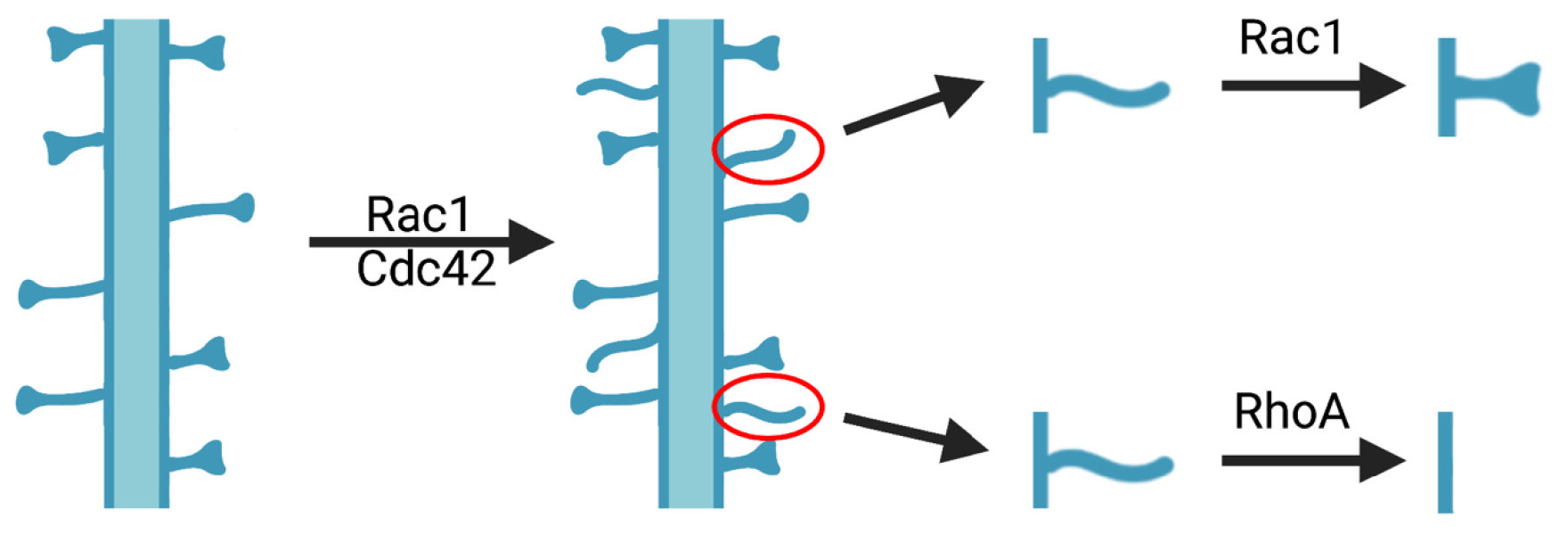
The Rho family GTPases Cdc42, Rac1, and RhoA are involved in the formation and maintenance of dendritic spines as shown here. Working together, Rac1 and Cdc42 cause new spines to form. Rac1 strengthens and stabilizes dendritic spines; RhoA promotes the pruning and eventual loss of dendritic spines.
Media Attributions
- mTORC flowcharts autism synaptic pruning © Jim Hutchins is licensed under a CC BY-SA (Attribution ShareAlike) license
- C4 in Synaptic Pruning and Autism © Aliyah Grijalva and Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- Transient vs Sustained Dendritic Spine Growth © Itzy Santillan is licensed under a CC BY-SA (Attribution ShareAlike) license
- cells-09-00835-g001 © Daji Guo, Xiaoman Yang, and Lei Shi is licensed under a All Rights Reserved license
- Rho signaling synaptic pruning autism © Haorui Zhang, Youssif Ben Zabiah, Haiwang Zhang, and Zhengping Jia is licensed under a CC BY (Attribution) license

