Quantal Release of Neurotransmitter and Evidence for Vesicular Release
Safina Rehman; Kynzie Lalliss; and Jim Hutchins
Chapter under construction. This is the first draft. If you have questions, or want to help in the writing or editing process, please contact hutchins.jim@gmail.com.
The Concept of a Synapse
Sherrington (1897)
The term synapse was introduced by Sir Charles Sherrington. This neologism was coined from the Greek word parts syn– and haptein, meaning “join together” or “clasp”. Sherrington, an English physiologist, is a pioneer in the field of neuroscience famously credited for his work regarding reflexes and the nervous system. While studying the activity of nerve cells, he used the word to describe the contact between two cells which played an important part in how they communicated with each other.
Loewi and Vagusstoff (1921)
In 1921, Otto Loewi discovered the neurotransmitter acetylcholine. The subsequent discovery of noradrenaline introduced the idea that cells transmitted information to each other using extracellular chemical signals. Loewi formed this hypothesis by taking two separate frog hearts and observing that by stimulating one, then collecting the fluid surrounding it and applying it to the second, the same effects occurred as if it had also been stimulated. This suggested to him that whatever chemical had been released in the first frog heart when it had been stimulated, was also responsible for effecting the second heart when it was exposed to the fluid containing that chemical. Not knowing what this chemical was, he called it Vagusreizproduct (“vagus stimulation product”) or Vagusstoff (“vagus substance”).
Dale’s Principle (1934)
Henry Hallett Dale furthered Loewi’s research, by identifying the vagus substance as the chemical acetylcholine. Dale tested the effects of different chemicals on the vagus nerve, which led him to observe that when acetylcholine was injected into the tissue, it mimicked the effects of the slowed heart rate that had been previously observed by Loewi in his experiments. This was a significant discovery because scientist had previously assumed nerves (brain cells) communicated only electrically, not chemically. It was then in 1936, Loewi and Dale were awarded the Nobel Prize for establishing the research of chemical synaptic transmission. This asserted that the activity of the nervous system was the result of neurons communicating with each other via chemical messengers.
From his research, Dale proposed that a neuron will release the same neurotransmitter for all of its communication with other neurons. For example, if a neuron releases acetylcholine, it can be assumed that it will not release a different chemical, and can then be defined by the chemical it uses to communicate. This hypothesis was called Dale’s Principle. It is often simply stated as, “one neuron, one neurotransmitter”. Neuroscientists no longer believe this is true. Today, Dale’s Principle is only of historical interest.
Electrical Properties of the Neuron
Hodgkin and Huxley (1952–1954)
In 1952 Alan Lloyd Hodgkin and Andrew Fielding Huxley used the squid giant axon, a large nerve fiber in the squid Loligo, to study how action potentials worked. At the time, it was known that nerve cells could generate electrical signals, but how it happened was still unclear. Hodgkin and Huxley conducted an experiment where they inserted a fine electrode into the axon (cell body) of a squid to measure the electrical potential (voltage) across it. They applied electrical stimuli to the axon and recorded the changes of the membrane’s electrical potential in response to it. During this they discovered that action potentials resulted from the movement of sodium (Na+) and potassium (K+) ions.
Resting Potential
Hodgkin and Huxley first measured the potential of the cell body while nothing was happening to it. This was called the resting potential. It was observed that during this, the distribution of potassium ions were abundant inside the cell and sodium ions were abundant outside.
Depolarization
After they stimulated the neuron, they observed that the membrane potential became more positive. This was a process called depolarization. This occurred due to the voltage-gated proteins embedded in the cell, opening up and allowing for the sodium ions outside of it to rush in. Sodium ions, having a positive charge, increased the electrical potential of the cell and made the resting potential rise.
Re-Polarization
After the electrical potential of the cell rose to its highest point, the channels that allowed sodium in closed, and channels that only allowed potassium through them, opened. This made for potassium to flow out of the cell and lower the electrical charge back to what it was at it’s resting potential.
All-or-Nothing Principle
This experiment showed that action potentials follow an all-or-nothing principle, meaning once the electrical potential of a neuron rose to its highest point, the action potential occurred all at once, this was due to the way the opening and closing of the embedded proteins in the cell body worked.
Chemical Synapse
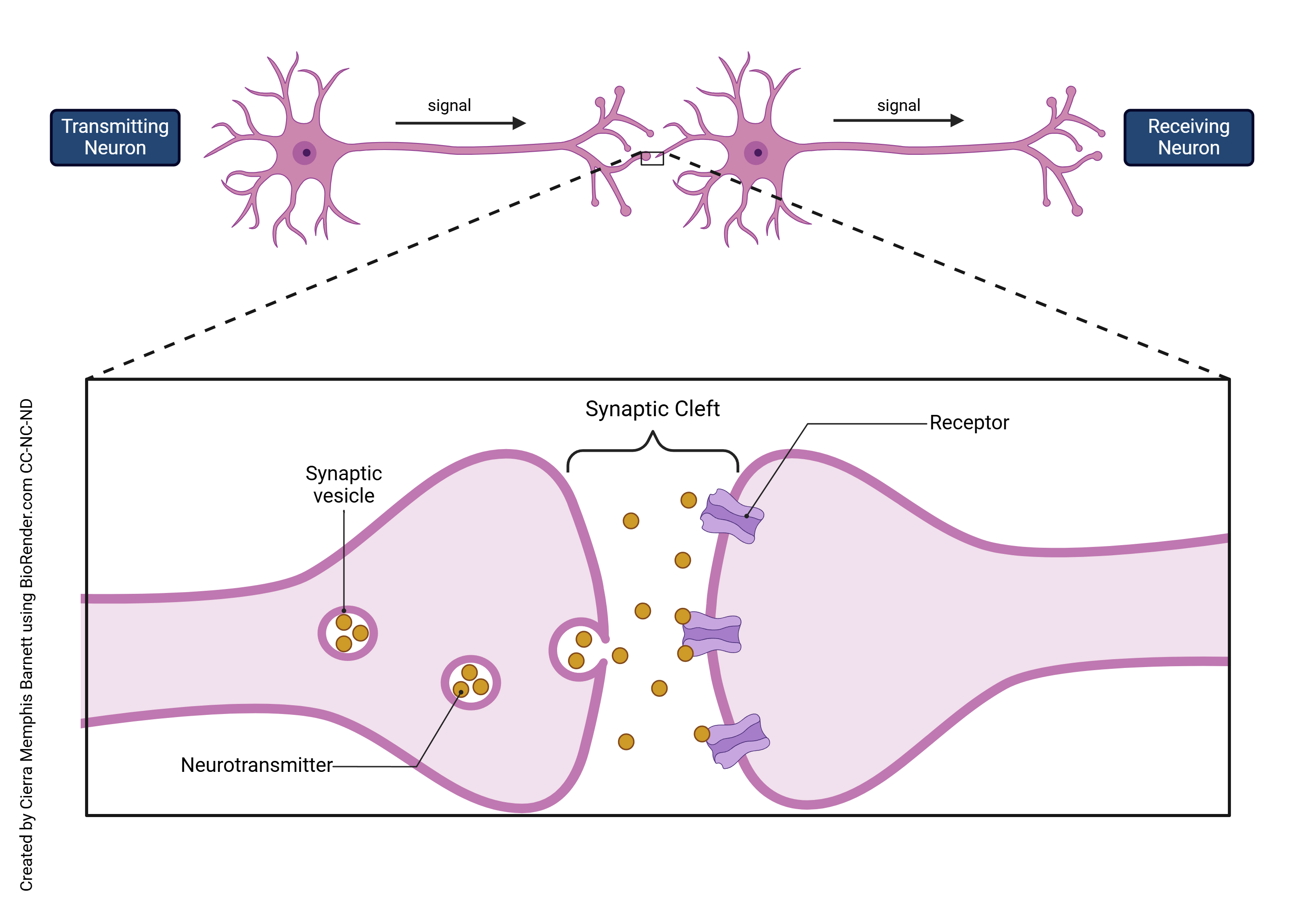
A chemical synapse is the term of art used by neuroscientists to describe a gap between two cells where an extracellular chemical signal (neurotransmitter) diffuses from the presynaptic cell to the postsynaptic cell. The presynaptic cell sends information in the form of a chemical neurotransmitter; a postsynaptic cell receives it. At the end of the cell body is the presynaptic terminal of a chemical synapse. Located here specifically are voltage gated calcium channels which open as a result of an action potential arriving after it has traveled down the neuron. Since there is no calcium in the cell, diffusion makes for the positively charged calcium outside to flow in. After calcium enters the cell, a process happens where neurotransmitters are released from synaptic vesicles, which are tiny sac-like organelles that have a lipid bilayer membrane like cells do. This process occurs when calcium binds to coil like structures called SNARE proteins. These proteins attach to both the membrane of the vesicle and the cell, then twists. This fuses the two membranes together to the extent that the vesicle opens up and dumps the neurotransmitters all at once into the synaptic cleft. After the neurotransmitters are dumped into the synapse (synaptic cleft), it binds to the receptors on the following postsynaptic cell. These receptors are often, but not always, embedded in the branch-like structures called dendrites, and when the signaling molecule binds to a receptor, they either cause an electrical change, or biochemical change, or both, in the postsynaptic neurons.
The Quantal Hypothesis
Fatt & Katz (1951)
Paul Fatt and Bernard Katz (1951) used the frog neuromuscular junction (NMJ) to study the details of neurotransmitter release. The NMJ is the synapse between the motor neuron presynaptically and the muscle fiber (muscle cell) postsynaptically. They recorded the membrane potential of the cells, and observed that when the presynaptic cell was electrically stimulated, it caused depolarization of the postsynaptic cell. The changes that occurred in the action potential of the postsynaptic cell are called end plate potentials (EPPs). These potentials arise because acetylcholine (ACh) is released from the presynaptic neuron and binds to receptors on the muscle cell membrane. The binding of ACh to its receptor, in turn, causes the ACh receptor to change shape, opening a gate in the membrane which allows Na+, K+, and Ca2+ ions to pass. The electrophysiologist observes the movement of these ions as a change in the voltage across the membrane. Even in the absence of presynaptic motor neuron stimulation, there were small, spontaneous EPPs. The spontaneous end plate potentials were much smaller than the original ones, so they called them miniature end plate potentials (MEPPs). Dozens of individual miniature end plate potentials, summed together, make up the EPP. Fatt and Katz reasoned that the MEPP is a packet or quantum of ACh. The end-plate potential seen in normal Ringer’s solution would then be the result of miniature ones added up. Each quantum corresponds to a fixed amount of ACh release.
Based on these observations, Bernard Katz proposed in 1952 that neurotransmitters are released not in a continuous flow, but in separate, same-size packets or “quanta“. This came to be known as the quantal hypothesis.
The Role of Calcium (Ca2+)
In 1952, del Castillo and Katz further studied the release of neurotransmitters in quantities by examining further the synaptic activity at neuromuscular junctions. It had already been discovered that neurotransmitters were not being released continuously but rather in fixed amounts, however it was still unknown how. By altering the concentration of calcium in the extracellular fluid of the synapse, they saw an effect on the size of end plate potentials, but not miniature end plate potentials. This meant that the small fixed amount in MEPPs was occurring in an all-or-none way. The fixed amount would be released entirely or not at all. The vesicles seen by early electron microscopists working at about the same time seemed to be a likely structure to hold a quantum of ACh.
The statistical analysis of the release of neurotransmitters could then represented by a Poisson distribution.
The Poisson Distribution
Siméon Denis Poisson, a French mathematician, developed the statistical distribution that carries his name. It was originally meant to represent the spread of a gambler’s probability of winning at gambling. A British actuary named R.D. Clarke developed this further to analyze bombings in London during WWII in order to create a statistical model that would prove German bombs were falling purely by chance and they didn’t possess accurate targeting mechanisms.
Del Castillo and Katz used this model for their experiment to represent the release of neurotransmitters because the size of consecutive end plate potentials were released in a random, not targeted, fashion, like German bombs. This meant the Poisson distribution was an ideal way of representing their data and the equation called Poisson’s law could predict the release.
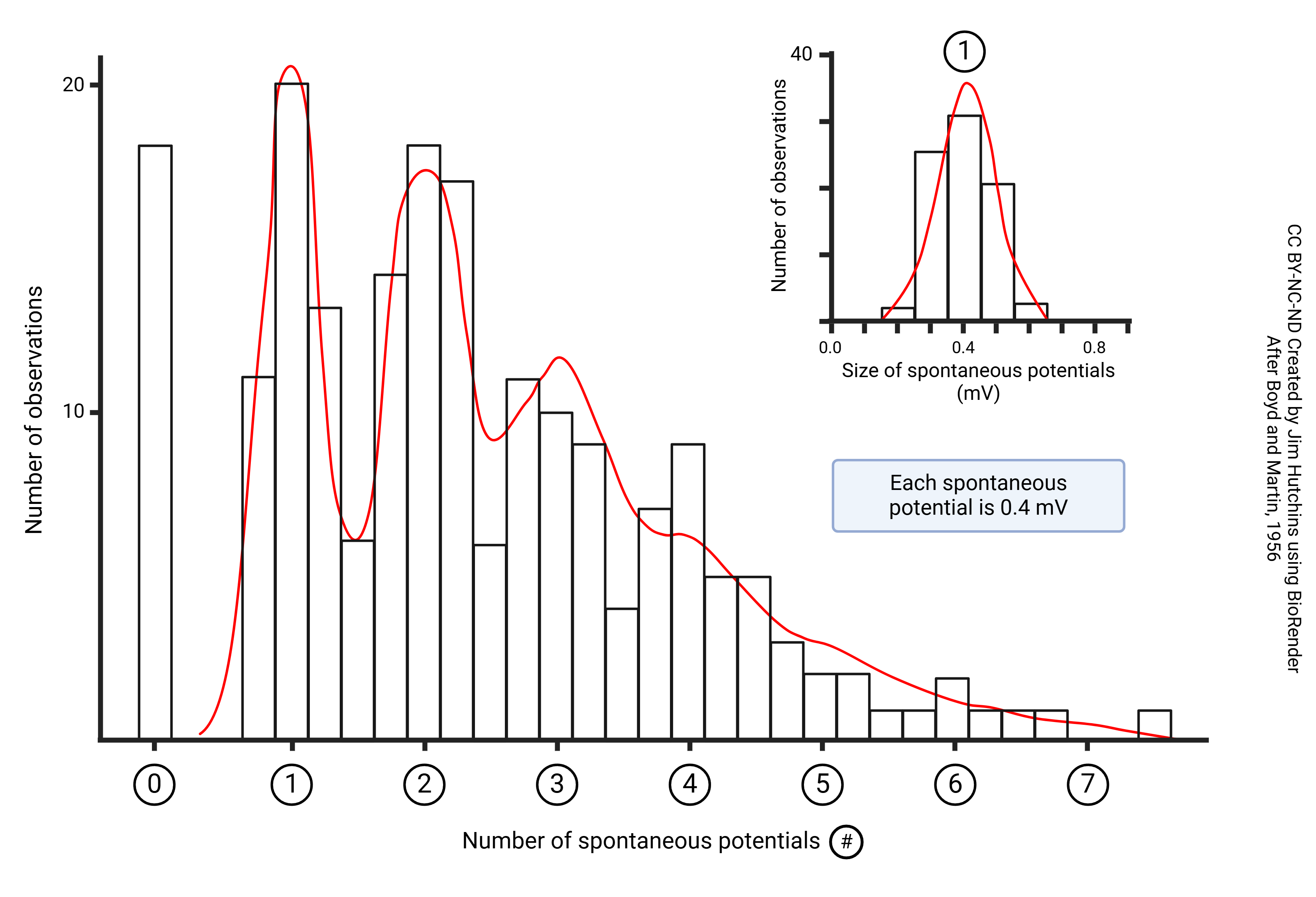
Boyd and Martin (1956) measured the size of spontaneous end-plate potentials in cat skeletal muscle bathed in a low-Ca2+ Ringer’s solution. Each vesicle released causes a depolarization of 0.4 mV. The number of vesicles released each time the motor neuron is stimulated is a good fit to the theoretical Poisson distribution (red line).
Miledi and Katz (1965)
Starting in the mid-1960s, Miledi and Katz discovered that if calcium was absent from the extracellular fluid of the cell, neurotransmitter release would not occur. This, combined with Katz’ previous observations, helped show that calcium had to be present in order for chemical synaptic transmission to occur.
The presence of calcium ions (Ca2+) is important for exocytosis to occur. Several experiments have shown that an increase in extracellular or manipulation of intracellular calcium levels affects exocytosis, either inhibiting or promoting it. Studies using calcium chelators or channel blockers also provides evidence for vesicular exocytosis and its dependency on calcium ion levels.
The Neuromuscular Junction
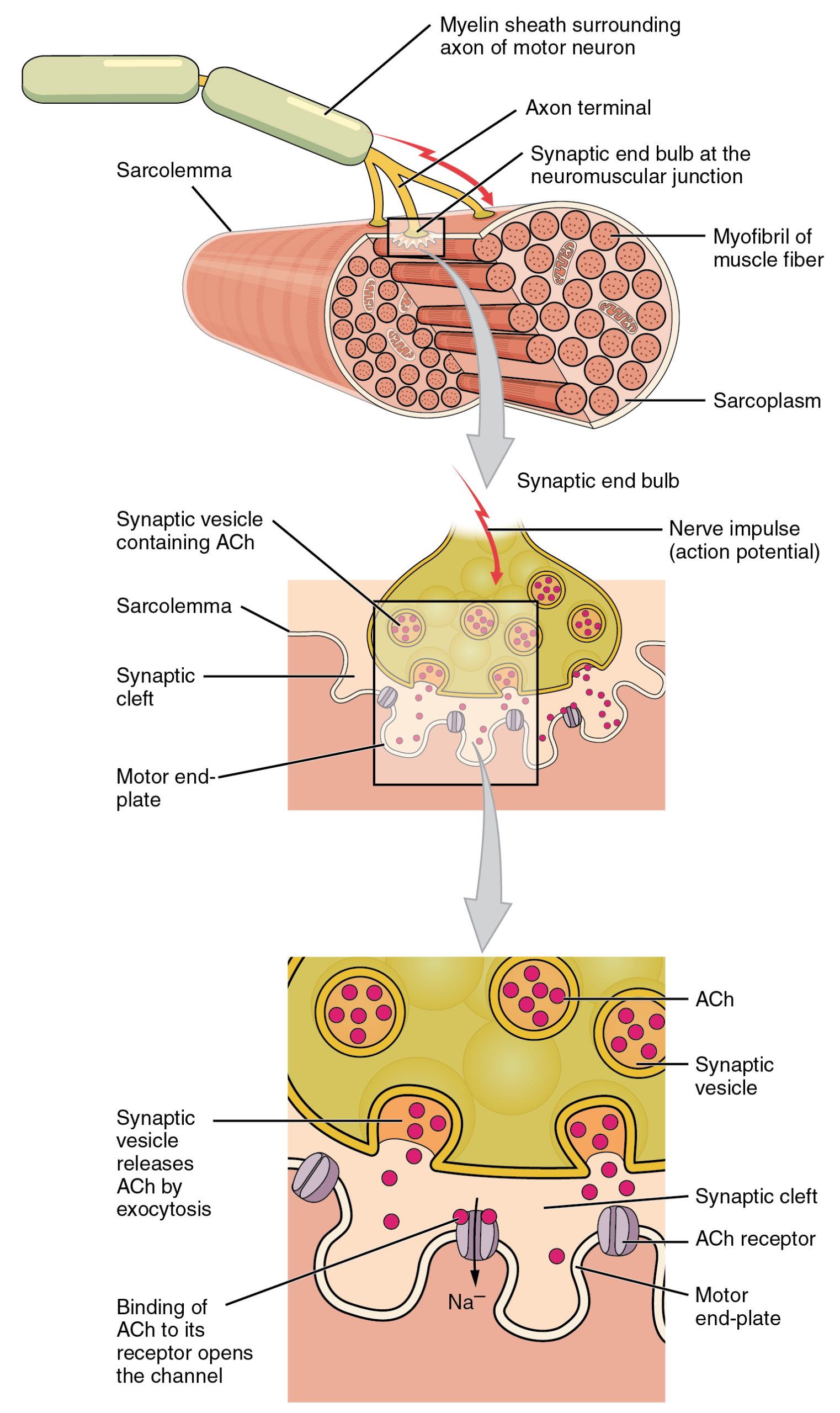 By 1950, physiologists knew that there were end-plate potentials in muscles (postsynaptic) at the neuromuscular junction (NMJ), the synapse between a motor neuron with its cell body in the spinal cord and a skeletal muscle cell.
By 1950, physiologists knew that there were end-plate potentials in muscles (postsynaptic) at the neuromuscular junction (NMJ), the synapse between a motor neuron with its cell body in the spinal cord and a skeletal muscle cell.
The electrical change in a neuron due to the process of chemical neurotransmission is called the excitatory postsynaptic potential (EPSP). The end-plate potential (EPP) is the equivalent concept at the neuromuscular junction.
The image shows the release of ACh (acetylcholine) from the vesicles of the motor neuron into the synapse between the cell and muscle fiber. Inside each vesicle in the neuron there is a fixed amount of acetylcholine which, when the vesicle opens, get dumped all at once into the space of the synapse. It binds to the rectors of the muscle fiber which allows for the contraction of the muscle fiber once the neurotransmitters have filled its receptors.
Vesicles
Transmission electron microscopy (TEM) came into full flower after the Second World War. This technique allows a resolution (ability to see small things) about 2000 times greater than light microscopy. Among the most interesting targets of electron microscopy was the neuron, and in particular the ribbon synapses of photoreceptor cells (discussed below). Palay and Palade 1955 demonstrated the presence of synaptic vesicles in a wide variety of neuronal types, while De Robertis and Franchi 1956 concentrated on the photoreceptor ribbon synapse.
The vesicles which were observed in initial studies of neurons by TEM provide direct evidence of vesicular exocytosis, as it shows detailed visualization of vesicles fusing with the cell membrane and releasing its contents into the extracellular space. This allowed scientists to see that the synapse had vesicles in which neurotransmitters were stored and released.
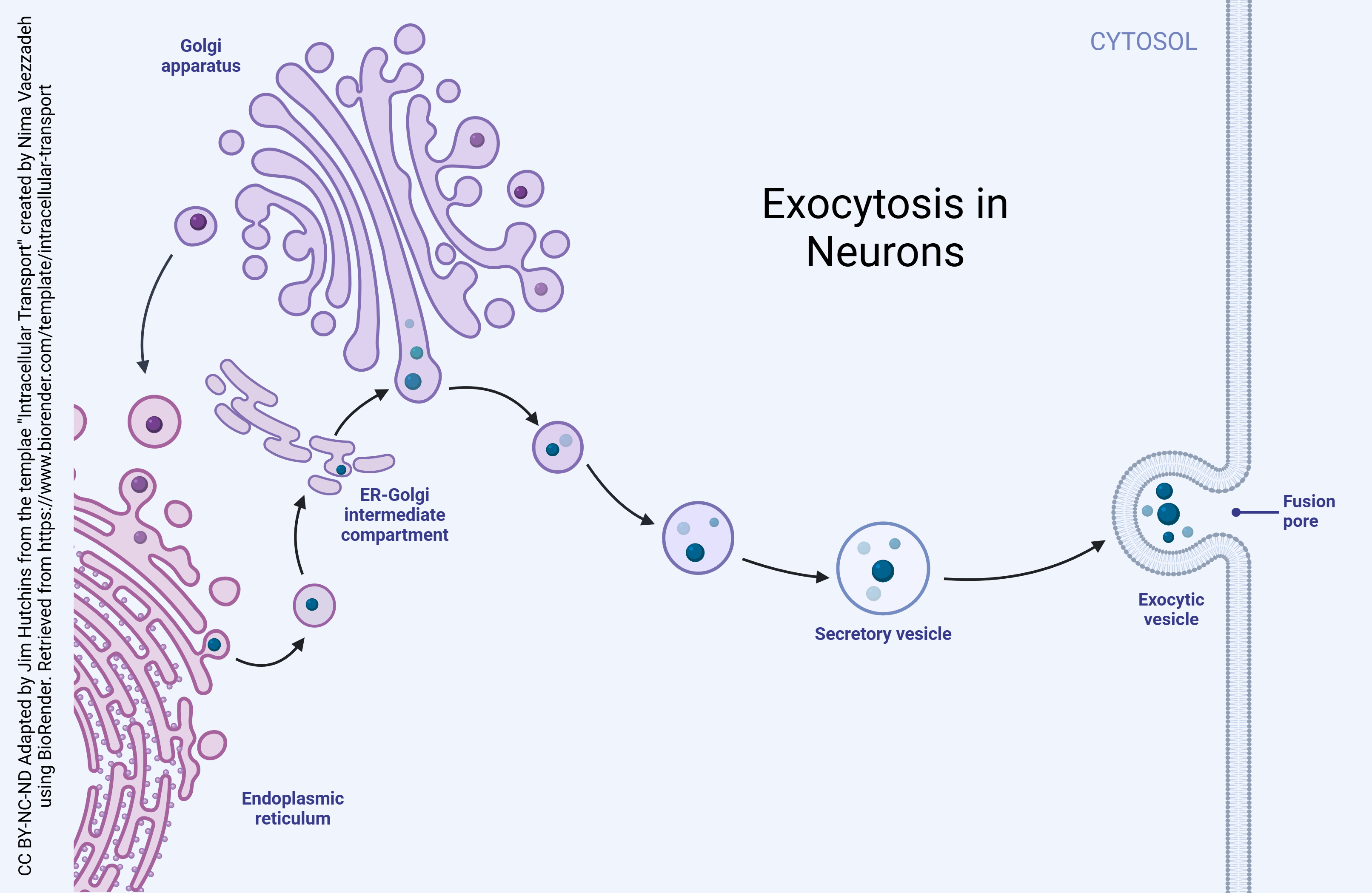
Exocytosis is the natural process through which molecules are transported from within a cell to the outside space of it. There are multiple different forms of exocytosis, but this chapter will focus on vesicular exocytosis. Vesicular exocytosis occurs when vesicles fuse with a cell’s plasma membrane to release their contents outside the cell. It is a form of active transport that allows a cell to move large molecules from within the cell to the exterior.
 A vesicle is a division within a cell that is formed by a lipid bilayer that separates its contents from the cell’s cytoplasm or extracellular fluid. These form naturally during processes of exocytosis, endocytosis, and the transportation of materials in the plasma membrane. Vesicles perform several different functions, mainly transporting materials and recycling waste. Additionally, they also absorb and dispose of toxic substances and pathogens within the cell to prevent cell damage and/or death.
A vesicle is a division within a cell that is formed by a lipid bilayer that separates its contents from the cell’s cytoplasm or extracellular fluid. These form naturally during processes of exocytosis, endocytosis, and the transportation of materials in the plasma membrane. Vesicles perform several different functions, mainly transporting materials and recycling waste. Additionally, they also absorb and dispose of toxic substances and pathogens within the cell to prevent cell damage and/or death.
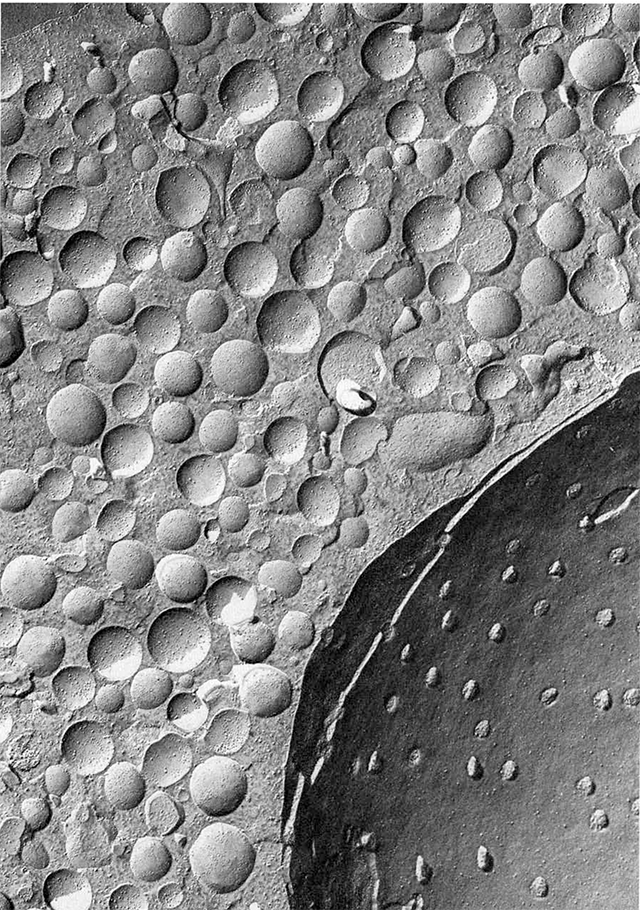
Freeze-fracture electron microscopy, as shown at left, involves the shattering of frozen tissue followed by a “sputter-coat” of metal to reveal the shapes of the frozen and fractured tissue. This technique reveals the structure of vesicles and also allows us to see the three-dimensional structure of both vesicles in the cytoplasm as well as vesicles caught in the process of releasing their contents.
These studies (see Heuser and Reese studies cited below) show pores forming as the vesicle fuses with the active zone of the presynaptic membrane.
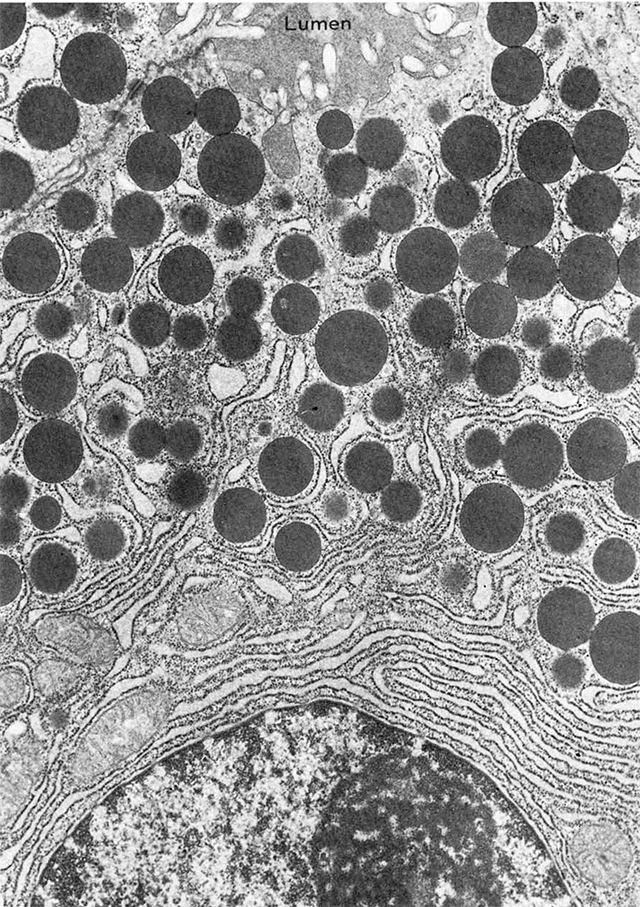 As visualized in transmission electron microscopy, vesicles can be clear or dense-core. Clear vesicles are now known to contain small-molecule neurotransmitters, while dense-core vesicles are filled with peptides which react strongly with osmium tetroxide (OsO4) used as a fixative and staining agent in electron microscopy. Examples of dense core vesicles are shown in the EM at right, along with the rough endoplasmic reticulum which synthesizes the contents of these vesicles.
As visualized in transmission electron microscopy, vesicles can be clear or dense-core. Clear vesicles are now known to contain small-molecule neurotransmitters, while dense-core vesicles are filled with peptides which react strongly with osmium tetroxide (OsO4) used as a fixative and staining agent in electron microscopy. Examples of dense core vesicles are shown in the EM at right, along with the rough endoplasmic reticulum which synthesizes the contents of these vesicles.
Patch-Clamp and Electrophysiological Studies
Patch-clamp and electrophysiological experiments in neurons have provided data that there are rapid changes in membrane potential that are associated with vesicle fusion and release. This study technique allows researchers to study ionic currents in cells, tissues, and patches of cell membrane, and directly measures membrane potentials. The dynamic nature of exocytosis is revealed with these studies, as well as evidence for the quantal release of neurotransmitters.
SNARE (soluble NSF attachments protein receptor) proteins, such as syntaxin, SNAP-25, and VAMP, are essential for the process of exocytosis and provide support for the concept on a molecular level. These proteins are the mediators for vesicle docking and fusion with a cell’s plasma membrane. When these proteins are tampered with and their function is disrupted, exocytosis is inhibited.
Capturing Vesicles Mid-Fusion
Heuser and Reese (1973)
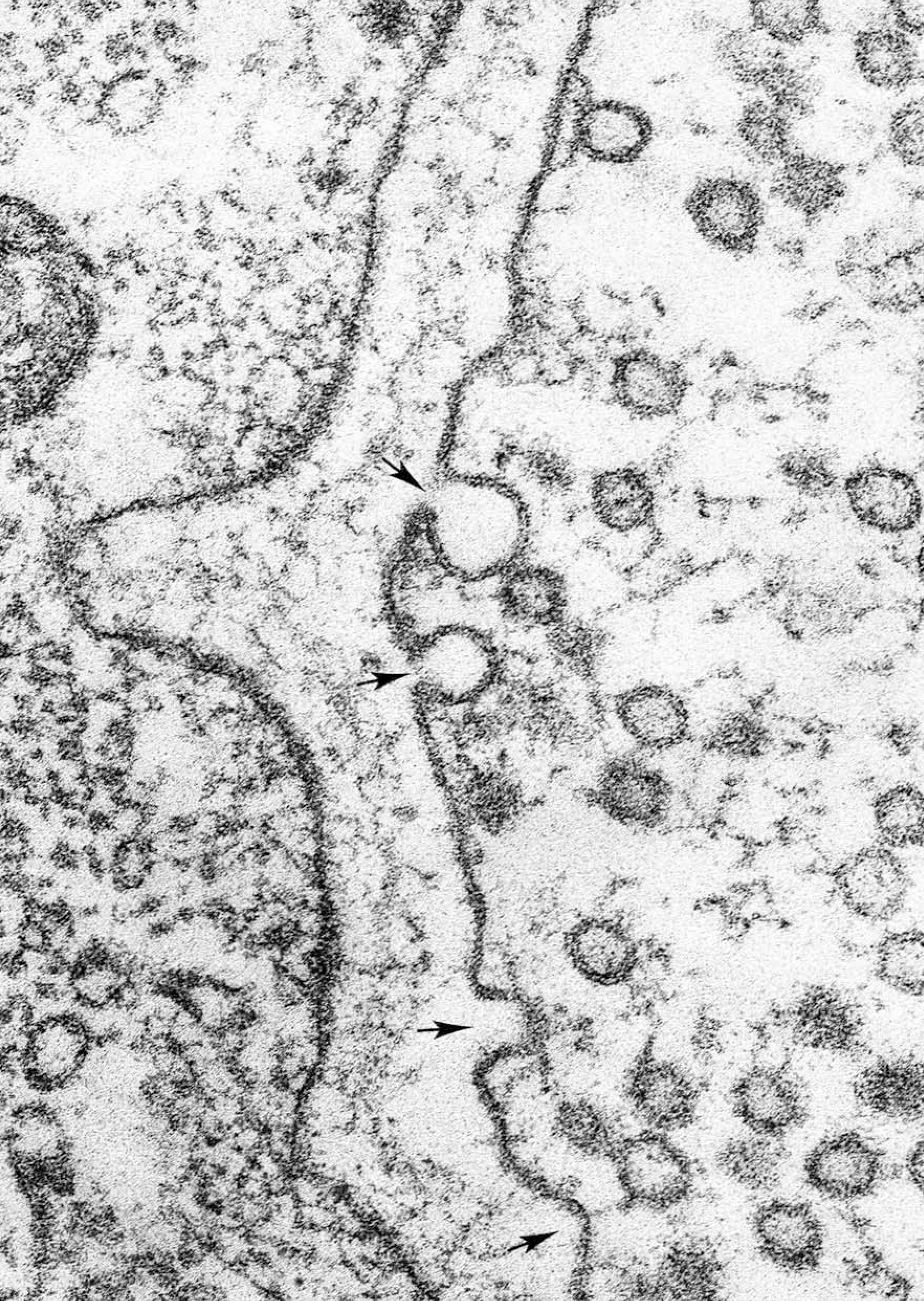
 Heuser and Reese observed the fusion of vesicles directly using freeze-fracture electron microscopy in the frog NMJ. They mounted a frog NMJ (with both pre- and postsynaptic elements) on a stage, then allowed the stage to rapidly drop towards a copper block cooled to liquid helium temperature (4K or –269°C) to rapidly freeze the preparation. Midway through its travel, the motor neuron was stimulated within milliseconds of slamming into the copper block. This caught the presynaptic membrane and vesicle mid-fusion.
Heuser and Reese observed the fusion of vesicles directly using freeze-fracture electron microscopy in the frog NMJ. They mounted a frog NMJ (with both pre- and postsynaptic elements) on a stage, then allowed the stage to rapidly drop towards a copper block cooled to liquid helium temperature (4K or –269°C) to rapidly freeze the preparation. Midway through its travel, the motor neuron was stimulated within milliseconds of slamming into the copper block. This caught the presynaptic membrane and vesicle mid-fusion.
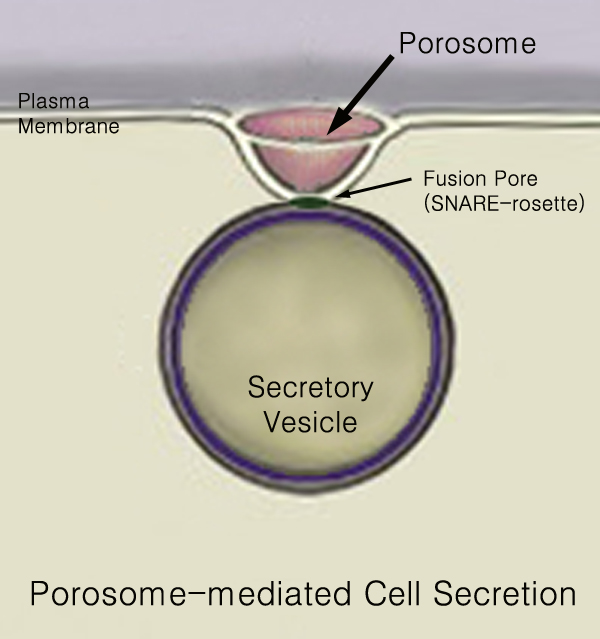
In conventional transmission EM, the fusion between vesicle and presynaptic membrane is termed an omega figure because of its resemblance to the Greek letter Ω. In freeze-fracture, as used by Heuser and Reese, the same structure (the porosome) is apparent as a pit in the presynaptic cell membrane.
Specializations Associated with Vesicles
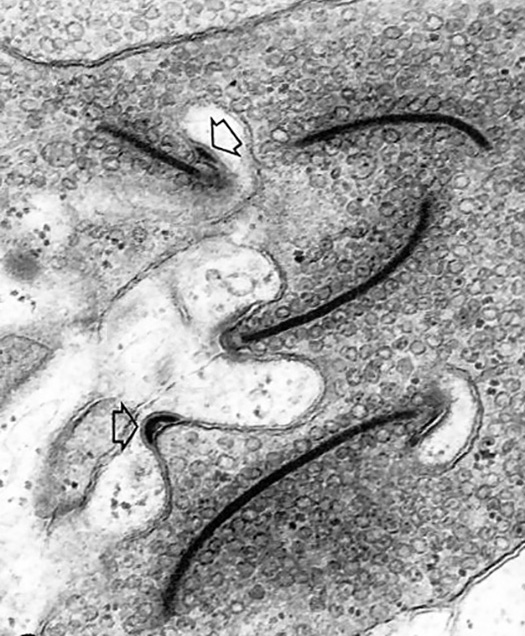
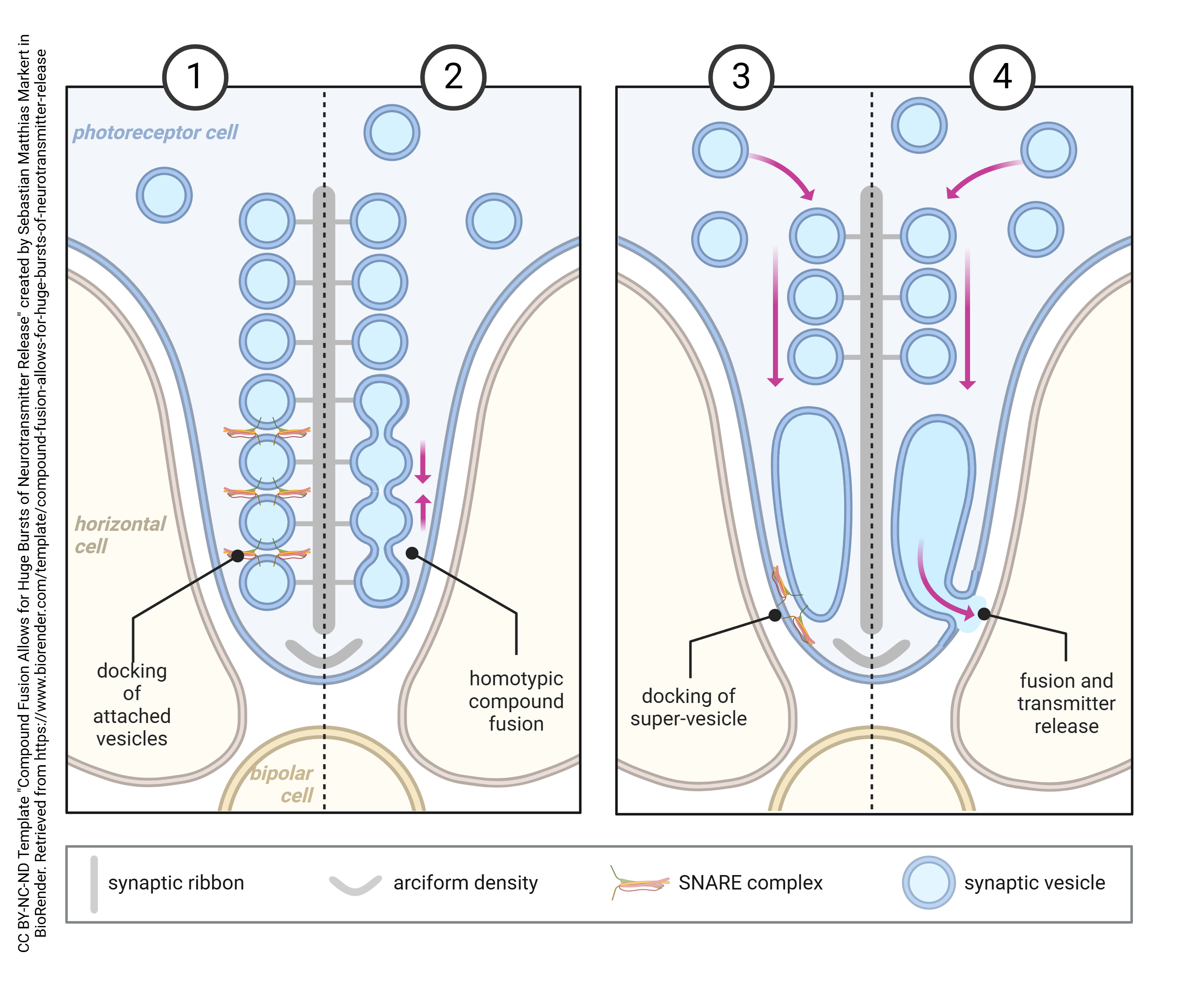
A further, independent line of evidence for vesicular release of neurotransmitter has been provided by the presence of specializations associated with the rapid, sustained release of neurotransmitter, for example at photoreceptor synapses where dark onset results in a massive release of glutamate.
These ribbon synapses were discovered in the initial EM observations of DeRobertis and Franchi (1956). Ribbons are large, electron-dense bars of protein, whose function was obscure for over 30 years.
Recently, it has been shown that synaptic ribbons serve as a way of lining up quanta so they can fuse together to form a super-vesicle made up of several dozen quanta all joined together intracellularly at the active zone, then released in a massive burst of glutamate when the photoreceptor is stimulated. (Weirdly, the release of glutamate is greatest in the dark, so this is when large bursts of neurotransmitter are released.)
 Radioactive Labeling of Neurotransmitters in Vesicles
Radioactive Labeling of Neurotransmitters in Vesicles
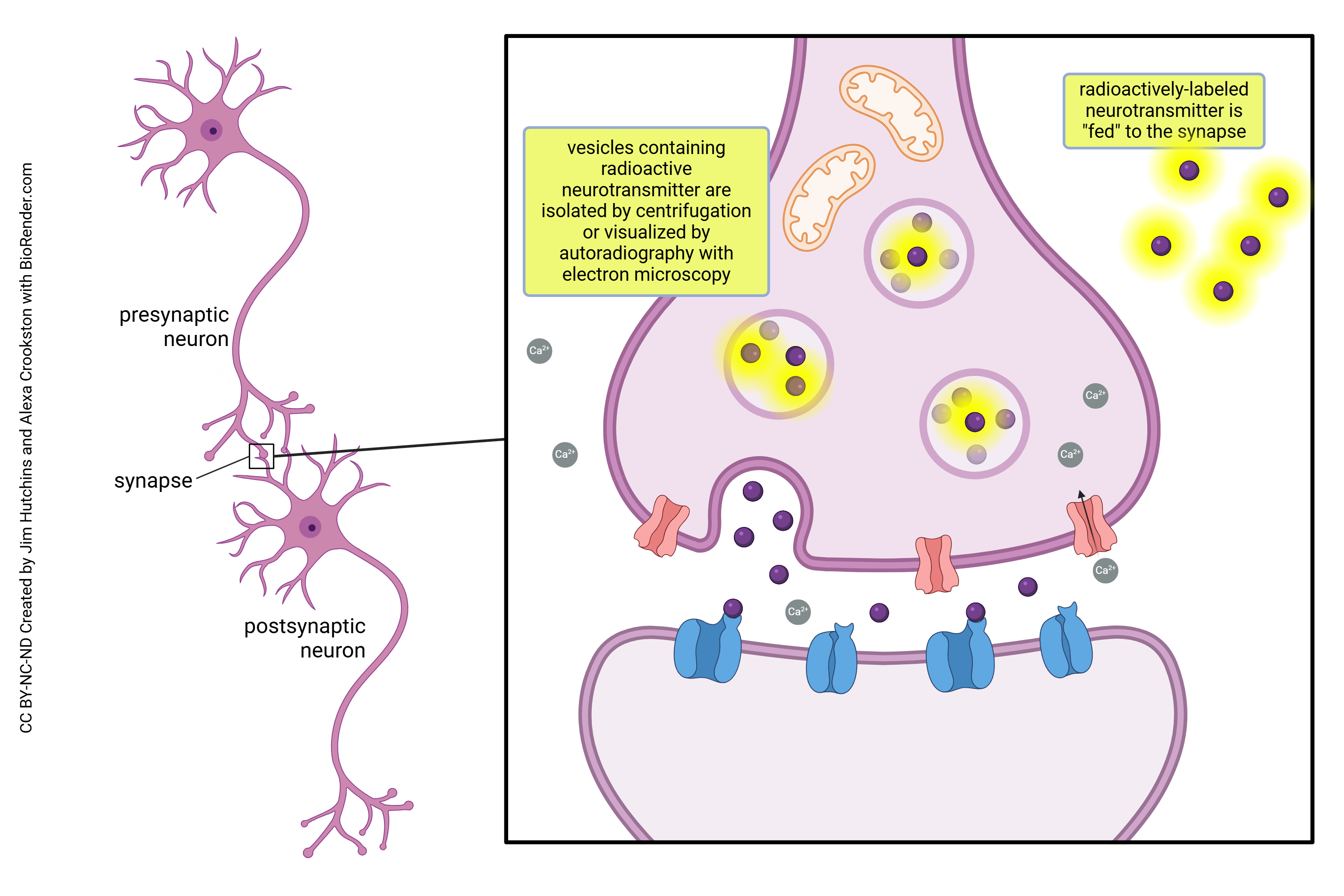
Researchers have used labeling techniques, specifically radioactive labeling, to trace the release of molecules during exocytosis. When using this type of imaging technique, researchers can see the tagged neurotransmitters moving between the inside of the vesicle space to the outside extracellular space. Further studies using fluorescent and radioactive tagging allows for real-time observation of both vesicle movement and fusion with the plasma membrane.
Almost-pure preparations of vesicles can be produced by centrifugation, or the presence of radioactive label in vesicles can be directly visualized through electron microscope autoradiography.
In these studies, the neurotransmitter can be tagged with a radioactive label (typically 3H, which emits a low-energy β particle that only travels a short distance) and allowed to incorporate into the tissue. Then, the excess label is rinsed away, and the preparation soaked in a fixative solution to hold the radioactively-labeled neurotransmitter in place. The specimen is then prepared as usual for conventional TEM. An extra step is the addition of a layer of photographic emulsion which contains silver halides (e.g. AgCl). When a particle resulting from radioactive decay strikes an AgCl molecule, a crystal of metallic silver is formed. This silver crystal is electron-opaque and therefore easily seen in the electron microscope hovering just over the location of the radioactive neurotransmitter. In this way, the presence of silver particles reveals the location of the labeled neurotransmitter molecules within vesicles.
Electrochemical Measurements
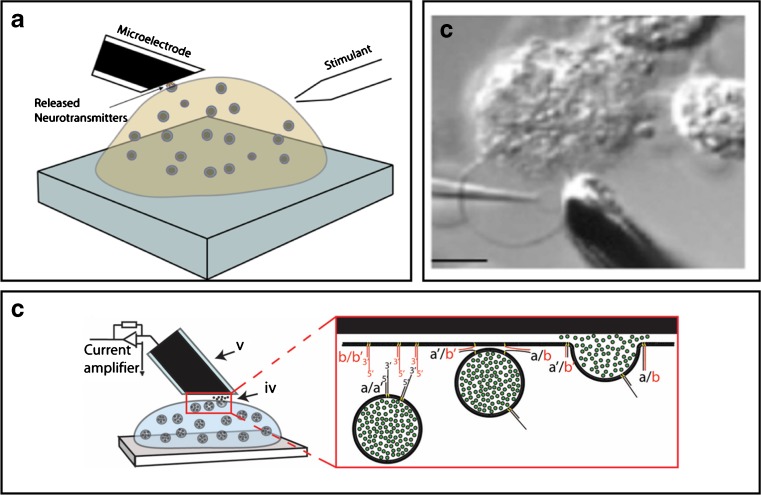 Amperometry is a method for measuring the electrons generated by chemical reactions between a charged neurotransmitter molecule and an electrode made of carbon or nickel. In this way, even the amount of neurotransmitter in the tiny volume of a vesicle (about 10–17 mL) can be analyzed.
Amperometry is a method for measuring the electrons generated by chemical reactions between a charged neurotransmitter molecule and an electrode made of carbon or nickel. In this way, even the amount of neurotransmitter in the tiny volume of a vesicle (about 10–17 mL) can be analyzed.
For example, Fathali and Cans (2017) have used amperometry has been used to detect vesicular release of dopamine, adrenaline, noradrenaline, and serotonin while Shadlaghani et al. (2019) report on the amperometric methods used to detect glutamate, choline, acetylcholine, and adenosine.
About 1 million catecholamine molecules are released during an average synaptic event. There are on the order of 10,000 neurotransmitter molecules in a single vesicle (quanta). Thus, about 100 vesicles fuse with the presynaptic membrane at the active zone when a neuron is stimulated.
Measurement of Membrane Capacitance
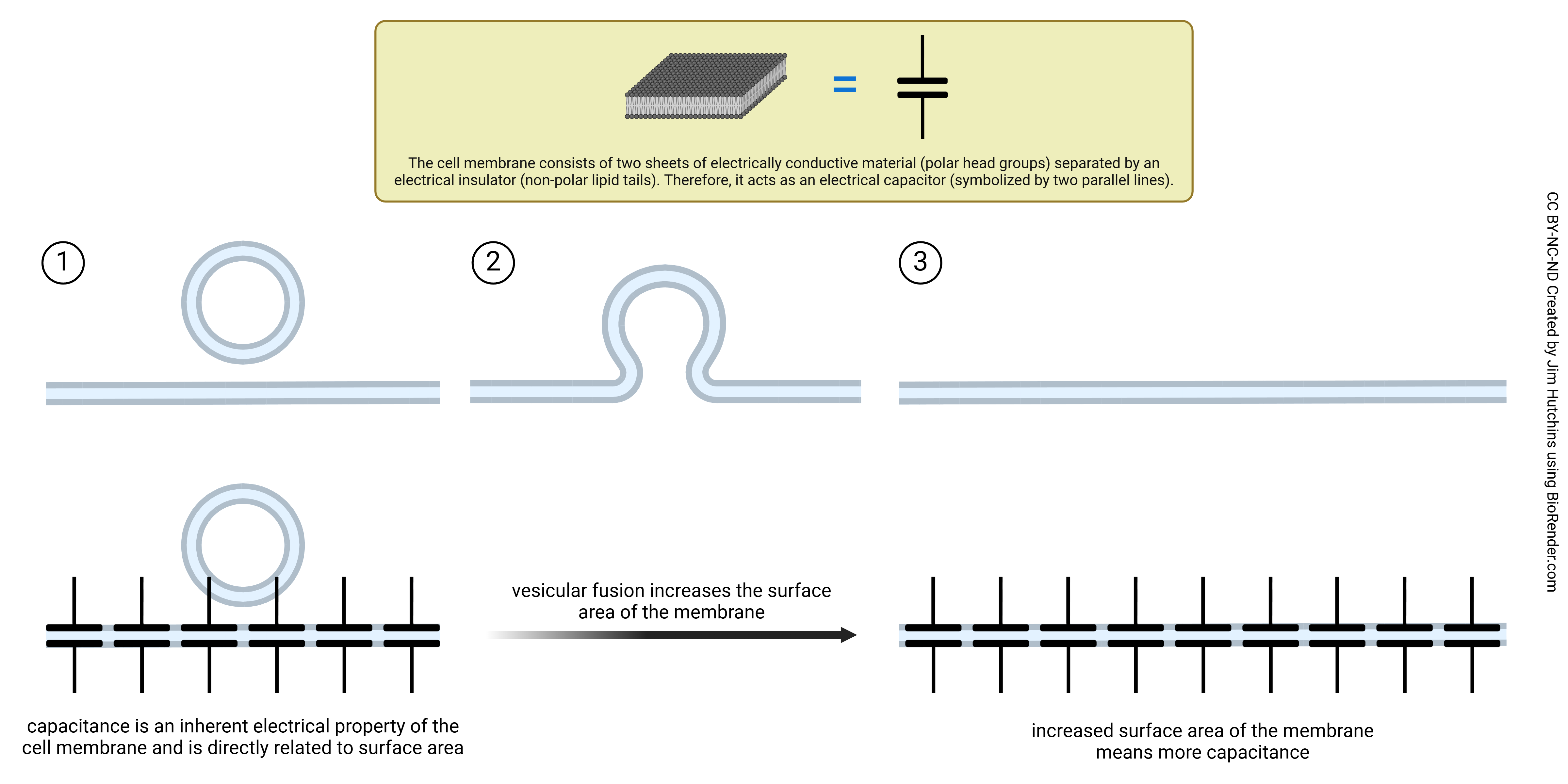 Capacitance is stored electrical charge across the lipid bilayer. In electrical circuit analysis, capacitance is symbolized by two charged plates (the horizontal lines) separated by an insulating layer (the gap between the paired horizontal lines). A neuronal cell membrane works the same way. The head groups form the charged plates. The lipid bilayer is composed of phospholipids which act as an insulator and creates an electrical capacitance, proportional to its surface area. Membrane capacitance influences how effectively neurons integrate incoming signals, affects the speed at which electrical signals travel along neurons, and impacts the rate at which neurons can fire action potentials. As vesicles fuse to the cell, it increases the surface area of the cell membrane which as a result, increases the amount of membrane capacitance. Each vesicle is about 40 nm in diameter. The surface area of a 40 nm sphere is 0.005 μm2. Each vesicle that fuses with the presynaptic membrane increases the surface area of a neuron by about 1/40,000 of its total (approximately 2000 μm2). This produces a tiny increase in the capacitance measured for the whole cell with an intracellular electrode. (A Farad, symbolized F, is a measurement of capacitance. In an electrical device, a typical capacitance would be about 10–6 F.)
Capacitance is stored electrical charge across the lipid bilayer. In electrical circuit analysis, capacitance is symbolized by two charged plates (the horizontal lines) separated by an insulating layer (the gap between the paired horizontal lines). A neuronal cell membrane works the same way. The head groups form the charged plates. The lipid bilayer is composed of phospholipids which act as an insulator and creates an electrical capacitance, proportional to its surface area. Membrane capacitance influences how effectively neurons integrate incoming signals, affects the speed at which electrical signals travel along neurons, and impacts the rate at which neurons can fire action potentials. As vesicles fuse to the cell, it increases the surface area of the cell membrane which as a result, increases the amount of membrane capacitance. Each vesicle is about 40 nm in diameter. The surface area of a 40 nm sphere is 0.005 μm2. Each vesicle that fuses with the presynaptic membrane increases the surface area of a neuron by about 1/40,000 of its total (approximately 2000 μm2). This produces a tiny increase in the capacitance measured for the whole cell with an intracellular electrode. (A Farad, symbolized F, is a measurement of capacitance. In an electrical device, a typical capacitance would be about 10–6 F.)
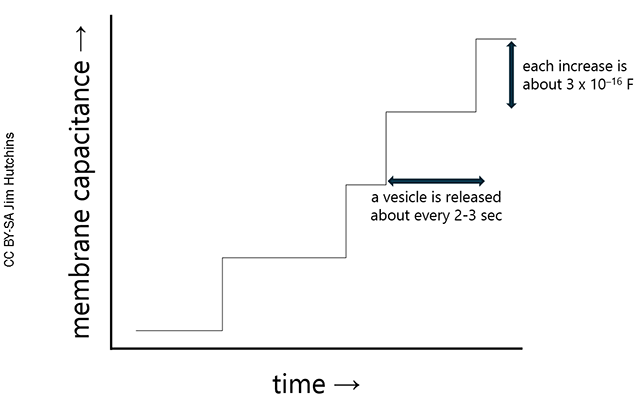
There is a stepwise increase in membrane capacitance with the fusion of each vesicle (Klyachko and Jackson, 2002). A representation of these results is shown at right. As a vesicle is released, there is a stepwise increase in membrane capacitance (about 3 x 10–16 F). This is about 1/10 billionth of the typical capacitance of an element in an electronic device.
Given time, the membrane would spread and the cell would become measurably larger as it continues to release neurotransmitter hundreds or thousands of times. To prevent this, there is a mechanism for pinching off the membrane and recycling empty vesicles.
Media Attributions
- Neuron Communication © Cierra Memphis Barnett is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- Poisson Analysis of Spontaneous Potentials © Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- Motor end plate © Betts, J. Gordon; Young, Kelly A.; Wise, James A.; Johnson, Eddie; Poe, Brandon; Kruse, Dean H. Korol, Oksana; Johnson, Jody E.; Womble, Mark & DeSaix, Peter adapted by Jim Hutchins is licensed under a CC BY (Attribution) license
- Exocytosis in neurons © Nima Vaezzadeh adapted by Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- U04-048 FawcettTheCell p694 © Don Fawcett is licensed under a All Rights Reserved license
- U04-049 FawcettTheCell p697 © Don Fawcett is licensed under a All Rights Reserved license
- Porosome © Taehee1016 is licensed under a CC BY-SA (Attribution ShareAlike) license
- FawcettTheCellChapter15-50 omega figures © Don Fawcett is licensed under a All Rights Reserved license
- jim masters thesis ribbon synapses © Jim Hutchins is licensed under a CC BY-SA (Attribution ShareAlike) license
- Compound Fusion Allows for Huge Bursts of Neurotransmitter Release © Sebastian Matthias Markert is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- Visualization of vesicular release with radioactively-labeled neurotransmitter © Jim Hutchins and Alexa Crookston is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- Amperometry methods © Hoda Fathali and Ann-Sofie Cans is licensed under a CC BY (Attribution) license
- Vesicle Fusion Increases Membrane Capacitance © Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- Vesicle release © Jim Hutchins is licensed under a CC BY-SA (Attribution ShareAlike) license

