Neurotransmitters: Peptides
Jaron Williams and Jim Hutchins
Chapter under construction. This is the first draft. If you have questions, or want to help in the writing or editing process, please contact hutchins.jim@gmail.com.
Neuropeptides
As highlighted in the previous chapters, neurotransmitters are as plentiful as they are critical. At times of increased neuronal excitement, neurotransmitters may need extra assistance to send signals. This is where neuropeptides, or sometimes referred to as neuroactive peptides, come into play. In this chapter, we will discuss the genesis, structure, and function of neuropeptides within the nervous system. As well as presenting a few specific examples of neuropeptides and their mechanisms of action in conjunction with neurotransmitters. Because the list of neuropeptides is extensive and ongoing, we will not be discussing every neuropeptide. For the purposes of this chapter we will provide an overview of the classification of neuropeptides with a few key examples to illustrate function.
Creation of Neuropeptides
Neuropeptides creation and structure are very similar to peptide neurotransmitters but differ in a few key ways. Firstly, it is important to note that neuropeptides are not considered to be neurotransmitters but rather are closer to chemical hormones. That being, said amino acid transmitters and neuropeptides do share some similarity in structure but differ in where they are formed. Amino acid transmitters are formed in nerve terminals from amino acids consumed from our diet, these active molecules are then stored in nerve terminals until they are released. In contrast, neuropeptides can only be synthesized in ribosomes in the dendrite of a neuron. These neuropeptides are then packaged into vesicles at the smooth endoplasmic reticulum (ER) and transported to nerve terminals for eventual release.
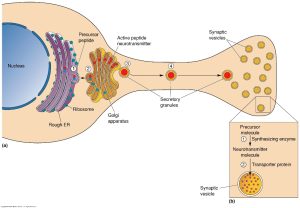
Peptide releasing neurons share several basic properties with chemically characterized neurons. Both of them store and release using vesicles and their release is dependent on Ca+ ions being present. Additionally, neuropeptides are mediated by G protein coupled receptors postsynaptically. The majority of neuropeptides are made by non neural secretory cells that line hollow viscera in the body, such as the stomach and intestinal tract. In fact, the first neuropeptides were discovered within the gut, working to help regulate the enteric nervous system. This discovery led to the term “gut-brain” connection being formed.
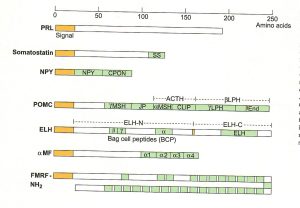
Each neuropeptides begins as a single amino acid precursor and is then processed post translation into an active peptide form. Some precursors can create multiple neuropeptides while others create just one. Each peptide family begins with a distinct precursor. The diagram below gives an outline of the possible neuropeptides that can be created from a precursor depending on the signal sequence present.
Function of Neuropeptides
Much of the function of neuropeptides is still being discovered, however there have been several important discoveries that have shed light on these important molecules. According to Dale’s law, neurons only release one neurotransmitter within a specific phenotype (cholinergic, adrenergic, etc.). Neurologists began to discover the presence of other neuroactive peptides along with neurotransmitters within the central and peripheral nervous systems. At first this was puzzling as there was often no need for additional neurotransmitters but it was later discovered that many neurons contain multiple neuropeptides in addition to their primary neurotransmitter, but why?
While the answer is not 100% clear, some examples within the autonomic nervous system have provided insight into what exactly neuropeptides do. According to these examples, when parts of the nervous system are experiencing a higher frequency of signals neuropeptides are released to supplement the primary action of the neurotransmitter. It may be helpful to think of neuropeptides as sidekicks to neurotransmitters, a batman and robin situation. When the primary hero is experiencing higher levels of difficulty (neurotransmitters) the sidekick (neuropeptides) can come in and help “save the day”. In the case of your nervous system, saving the day could look like increasing vasodilation or helping block pain receptors.
Neuropeptide Release
While both neuropeptides and neurotransmitters are released via vesicles at the synapse the exact location and release of them differ. Neurotransmitters are released using small vesicles within the active zone which leads to a quick release. Neuropeptides are released relatively far from the active zone and are done so using larger sized vesicles. This combination leads to a much slower release when compared to neurotransmitters. In addition to the distances and size of vesicles neuropeptides also need a much stronger entry signal from Ca++ in order to elicit a reaction. This is partially why the body works by using neurotransmitters first and the supplements reactions with neuropeptides. The following image provides a visual representation of the differences in size and location of release.
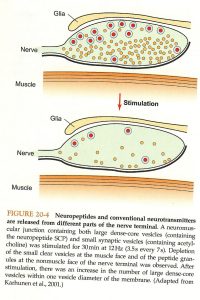
This mechanism of action does help with prolonging the effect of specific neurotransmitters. While many neuropeptides supplement a neurotransmitter signal, some work simply by increasing the length for which the effect takes place. With neuropeptides being released at a slower rate they are able to extend certain reactions when needed.
Neuropeptides in the Salivary Glands
Let’s take a look at the action of neuropeptides in the salivary glands as an example. When the parasympathetic nerves are stimulated at low levels, neurons release acetylcholine in order to increase salivation. ACH accomplishes this by activating muscarinic receptors leading to the release of saliva. When these same neurons become more frequently stimulated we also see the release of vasoactive intestinal peptide or VIP. VIP is a strong vasodilator which helps to increase glandular blood flow, thereby increasing salivation. We can see in this instance that ACH and VIP work simultaneously, with different mechanisms of action, to increase the same reaction within the body. ACH being the “main hero” and VIP being the “sidekick”. In this specific instance VIP works by amplifying the signals of being sent by ACH, as discussed previously some interactions between neurotransmitters and neuropeptides simply extend the length of a signal.
Media Attributions
- IMG_0498 (1)
- 97856
- 17592

