Neurotransmitters: Glycine
Lance Castro and Jim Hutchins
Chapter under construction. This is the first draft. If you have questions, or want to help in the writing or editing process, please contact hutchins.jim@gmail.com.
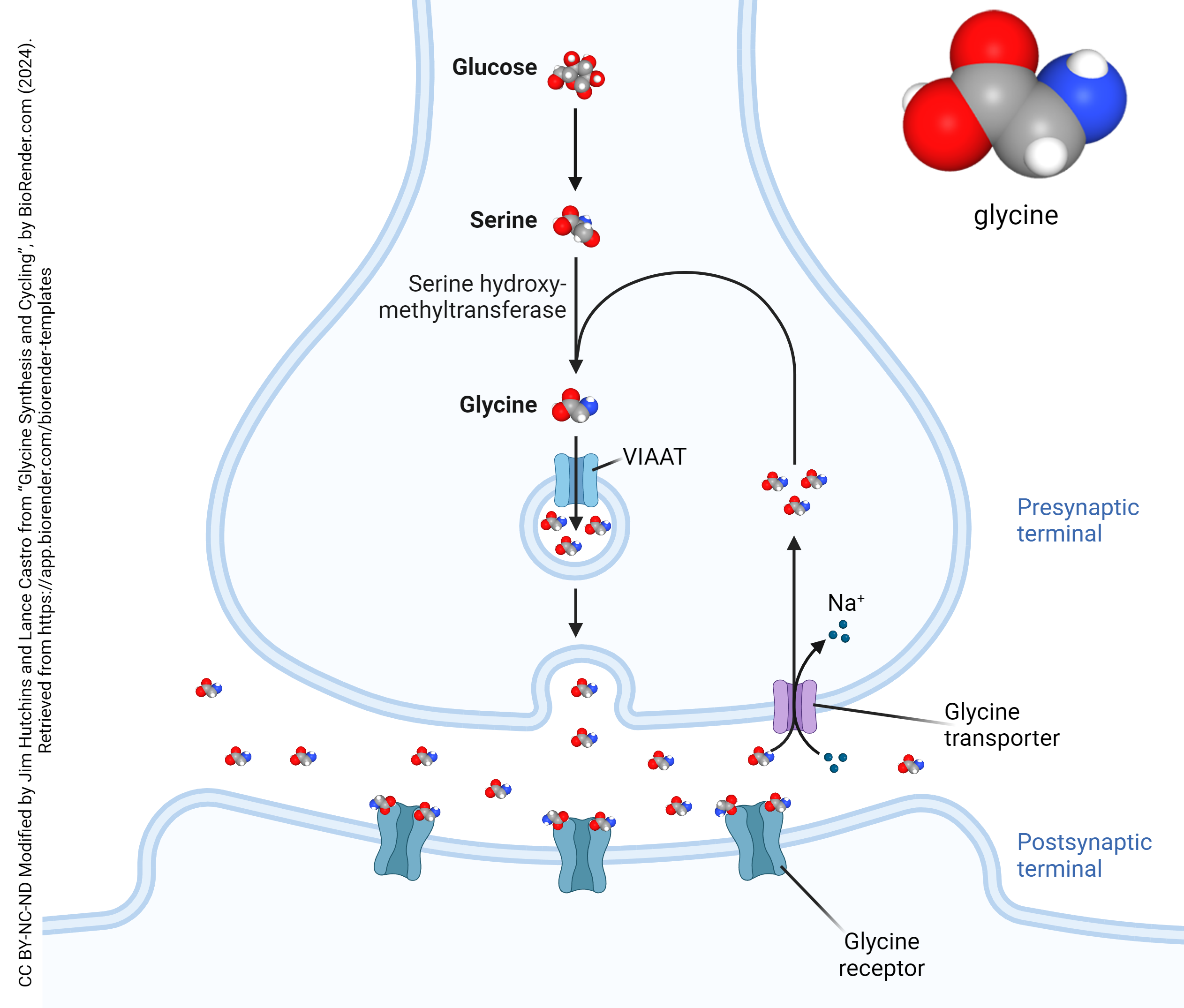
Glycine Synthesis
Glycine is the simplest amino acid, and its immediate precursor is serine, which is converted from glucose via the serine synthesis pathway (SSP). Glycine is synthesized from serine at the presynaptic terminal by the actions of the enzyme serine hydroxymethyltransferase (SHMT). From there, it is packaged into small vesicles bound for the synaptic cleft by the vesicular inhibitory amino acid transporter (VIAAT).
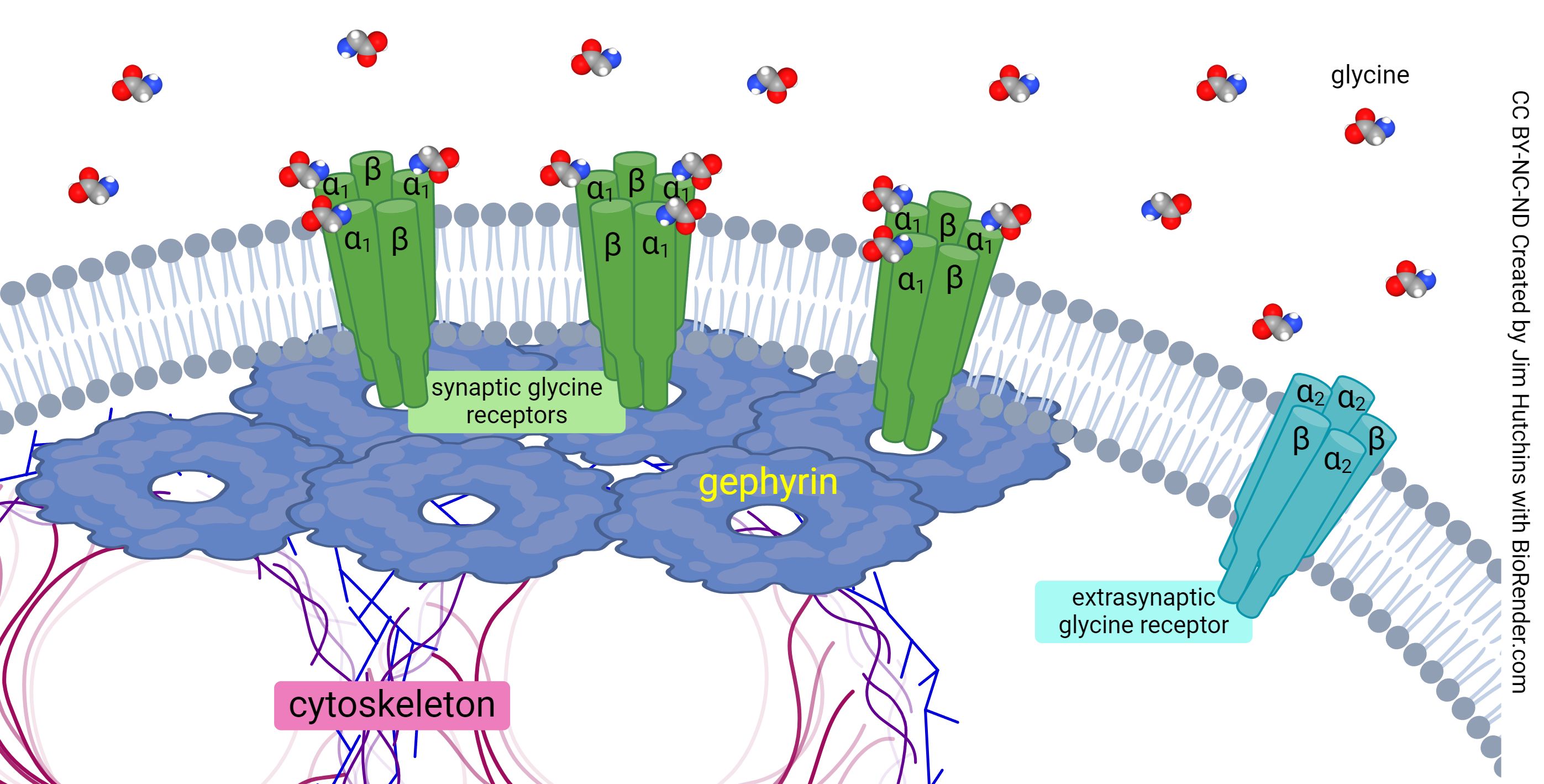
The Glycine Receptor
Glycine’s receptor (GlyR) can be activated by a number of amino acids, with varying levels of effectiveness, though its greatest affinity is to glycine itself. The rat poison strychnine is a glycine receptor antagonist. Three molecules of glycine are required to activate this receptor. The glycine receptor is an ionotropic chloride channel, and as such its activation results in hyperpolarization and subsequent inhibition of the postsynaptic cell. It has a pentameric structure, consisting of three α proteins and two β proteins. Because there are three α proteins and three glycine binding sites, it is assumed that the α protein contains the glycine pocket. There are four types of α subunit (α1 – α4) and one type of β subunit.
The Function of Glycine

Physicians of the 18th and 19th century noted the similarities between tetanus and strychnine poisoning but the molecular basis for that connection necessarily waited for a late 20th century understanding of the pharmacology and circuitry of the glycinergic system in the spinal cord. The toxin produced by the bacterium Clostridium tetani (which occupies infected wounds) interferes with the vesicular release of neurotransmitters including glycine in the spinal cord. Strychnine is a glycine receptor antagonist.
For the first half of the 20th century, a debate started by Cajal and Golgi raged on. Sir Henry Dale and others proposed the existence of synapses, with the transfer of a chemical substance between neurons, as proposed by Cajal. Sir John Eccles initially believed in a direct electrical connection between neurons, an idea first proposed by Golgi.
Brock, Coombs, and Eccles (1952) began recording from motor neurons in the spinal cord using intracellular glass electrodes, a novel technique at the time. They found inhibition of motor neurons that could not be explained by the electrical/syncytium hypothesis; therefore it was abandoned. Now the search was on for the wiring and chemistry underlying inhibition of spinal cord motor neurons (α motor neurons or lower motor neurons).
In 1965, Aprison and Werman published a study on the neurochemistry of the spinal cord and spinal nerve roots of cats. Within this study, it was noted that the concentration of glycine was much higher in the spinal cord when compared to the rest of the CNS. This provided supporting evidence for glycine as a neurotransmitter in the spinal cord. Subsequent electrophysiological investigation (e.g. Curtis et al., 1976) using strychnine showed that the Renshaw cell, a glycinergic interneuron, was a key element of two kinds of inhibition producing rhythmic behavior: the reciprocal pathway which depends on sensory input from muscle spindle receptors to shut off the α motor neuron; and the recurrent pathway, where an α motor neurons sends an axon branch to activate a Renshaw cell, which releases glycine onto the α motor neuron and shuts it off.
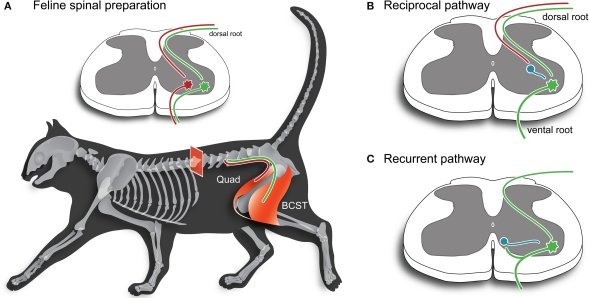
Glycinergic interneurons can also be found in the retina, auditory systems, the hippocampus, and other neural structures responsible for processing sensory information.
Termination and Breakdown
Glycine action is terminated either by reuptake into the presynaptic cell, where it is repackaged into vesicles for later release, or through transport into glial cells where breakdown can occur via the glycine cleavage system. The glycine cleavage system is a multienzyme complex that is composed of four different proteins; three enzymes and one carrier protein. The enzymes are known as P-protein, T-protein, and L-protein, respectively, while the carrier protein is known as H-protein. Within animals, including humans, the glycine cleavage system is confined to the mitochondrial inner membrane, where it functions to catabolize glycine into a methylene group, carbon dioxide, and ammonia. Deficits in this cleavage system are proposed to be the cause of nonketotic hyperglycemia, which is an inborn metabolic disorder characterized by abnormally high glycine levels in blood, urine, and cerebrospinal fluid. Due to the higher concentration of glycine being primarily present within the brain and spinal cord, many symptoms are neurological in nature, though hypotonia is often present.
Media Attributions
- The Glycinergic Synapse © Lance Castro and Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- Anchoring of the glycine receptor at the synapse © Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- Opisthotonus in a patient suffering from tetanus © Sir Charles Bell is licensed under a Public Domain license
- Cat hindleg © Robert John Callister and Brett Anthony Graham is licensed under a CC BY (Attribution) license

