Neurotransmitters: Glutamate
Lindsey Aune and Jim Hutchins
Chapter under construction. This is the first draft. If you have questions, or want to help in the writing or editing process, please contact hutchins.jim@gmail.com.
Glutamate Production and Packaging
Glutamate (the ionized form of glutamic acid) is found in proteins; it is not particularly common, making up about 5.8% of the 20 amino acids (average would be 5.0%).
As a signaling molecule, however, it is quite common. About 40% of the synapses in the brain release glutamate, and about 90% of the neurons respond to it (that is, have some kind of glutamate receptor).
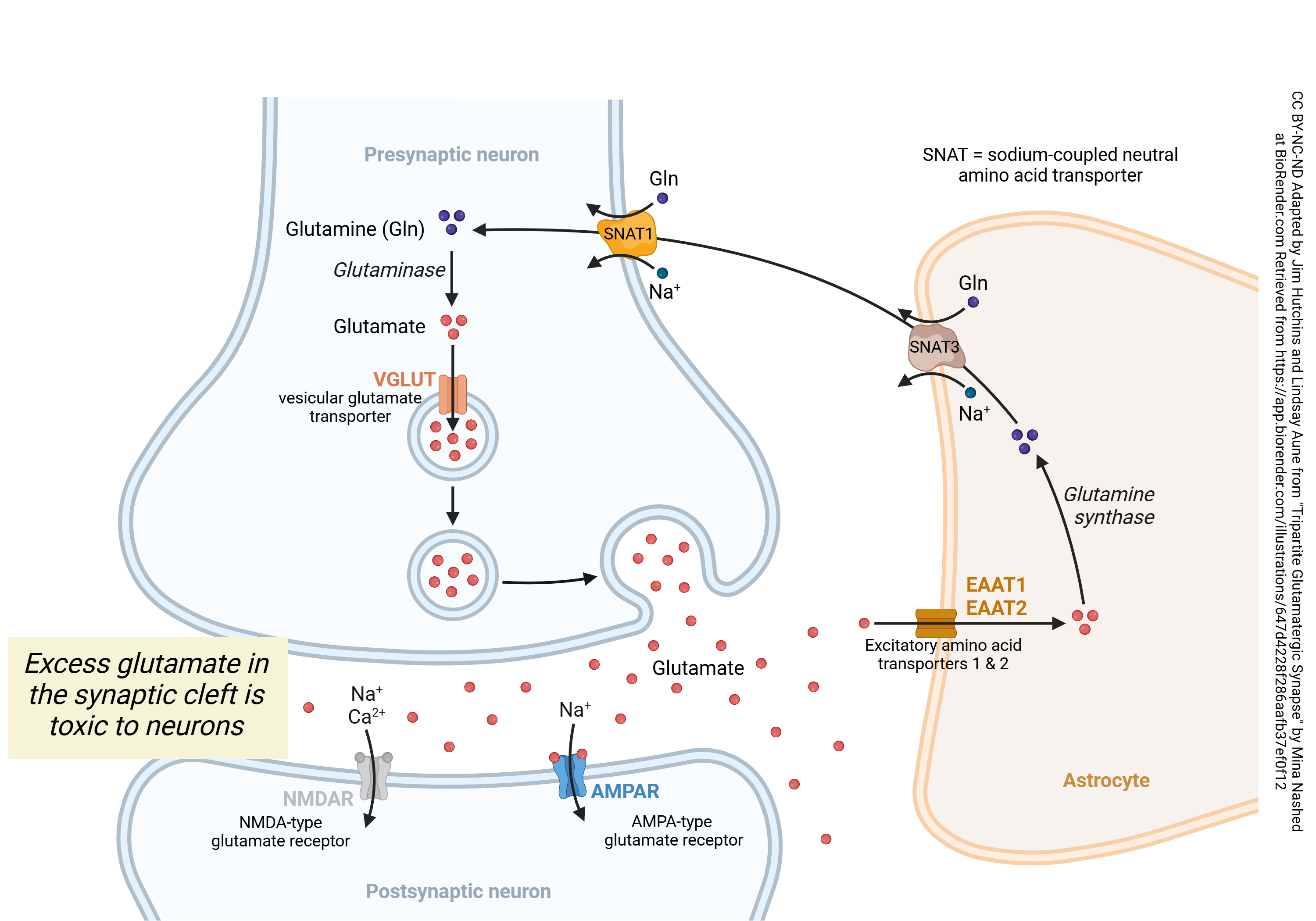
When glutamate is released from synaptic terminals, it diffuses across the synaptic cleft to interact with receptors on the postsynaptic surface. Effective signaling requires a way of stopping the action of glutamate. This happens because the excitatory amino acid transporter 1 (EAAT1) or 2 (EAAT2) latches on to the glutamate molecule and pulls it into an astrocyte. The enzyme glutamine synthase replaces the –COOH (carboxylic acid) group at the end of the amino acid –R group with a –CONH2 (amide) group. As shown below, this renders the molecule harmless; it can no longer bind to the glutamate receptor. The sodium-dependent neutral amino acid transporter (SNAT) 2 expels this glutamine from the astrocyte. It is then taken up by SNAT1, which is a co-transporter (symport protein) which carries in a Na+ ion along with the glutamine molecule. (Note that the Na+ ion must subsequently be pumped out by the Na+/K+ pump, an ATPase, so the SNAT1 transporter increases the energy requirement of the neuronal cell.) The enzyme glutaminase then converts glutamine back to glutamate, and the vesicular glutamate transporter (VGLUT) concentrates glutamate in acidic vesicles by exchanging glutamate for a proton (i.e., a hydrogen ion, H+). Because the vesicle must be made acidic by a proton pump for the VGLUT to operate, the packaging of glutamate into vesicles is also an energy-requiring process. Glutamate is released from synaptic vesicles in the usual manner, as described elsewhere.
Postsynaptic Glutamate Receptors
Ionotropic glutamate receptors include:
- α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors
- N-methyl-D-aspartate (NMDA) receptors
- kainic acid receptors
Metabotropic glutamate receptors include eight different types (and many different subtypes). The main types are abbreviated mGluR1 through mGluR8.
The AMPA Glutamate Receptor
The α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor is the most common of the glutamate receptors. It is an ionotropic receptor. That is, when glutamate binds to the AMPA receptor, the shape of the receptor protein changes, opening a channel for Na+ ions. Because Na+ flows down its concentration gradient from outside to inside, this depolarizes the neuron.
The NMDA Glutamate Receptor
As explained in Introduction to Neuroscience, NMDA glutamate receptors are coincidence detectors that play a key role in the processes of learning and memory.

Glutamate released from the presynaptic terminal diffuses across the synaptic cleft and binds to the AMPA receptor, opening a ligand-gated sodium ion (Na+) channel. This slightly depolarizes the postsynaptic neuron.
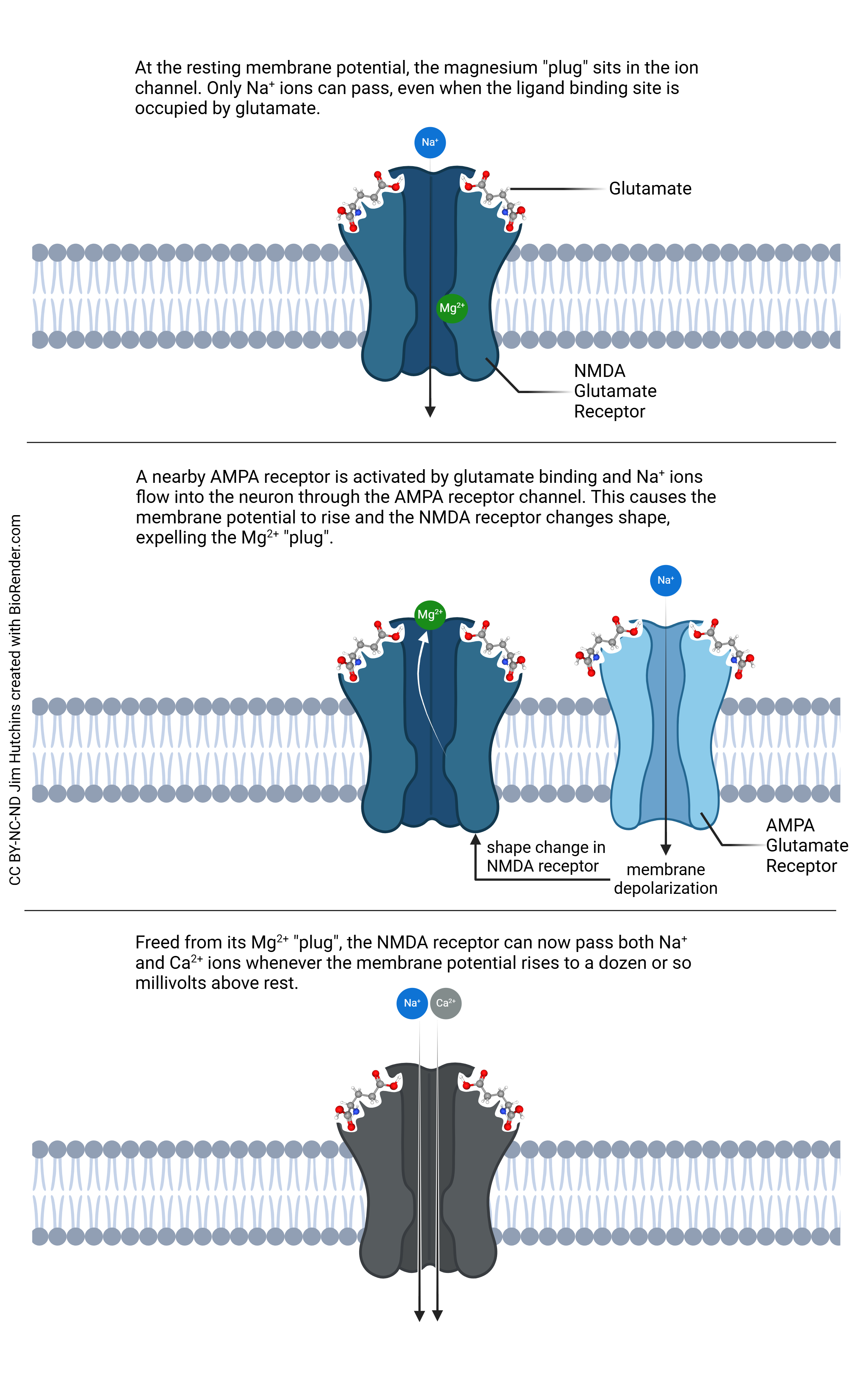
In contrast to the simple ionotropic action of the AMPA receptor, the NMDA receptor has a more complex mechanism. The neuron holds a voltage across the cell membrane, even when it’s not active. This is called the resting membrane potential. At or near the resting potential, the NMDA receptor operates like the AMPA receptor: it only allows the flow of Na+ ions. Unlike the AMPA receptor, however, the NMDA receptor has a Mg2+ “plug”. When the voltage rises from the normal, negative value, the Mg2+ plug is displaced and now, the NMDA receptor can allow calcium ions (Ca2+) to flow into the neuron.
A small amount of Ca2+ entry results in the activation of key intracellular signaling pathways such as those needed for the strengthening of synapses in learning and memory processes. But when nearby neurons die, the uncontrolled flood of glutamate and the larger amount of Ca2+ entry through the NMDA receptor which results kills neurons. Thus, a small amount of glutamate is essential; a slightly larger amount is deadly. It’s clear the neuron and astrocyte need to work together to limit the amount of glutamate that is available within the synaptic cleft.
How Does Glutamate Bind to the NMDA Receptor?
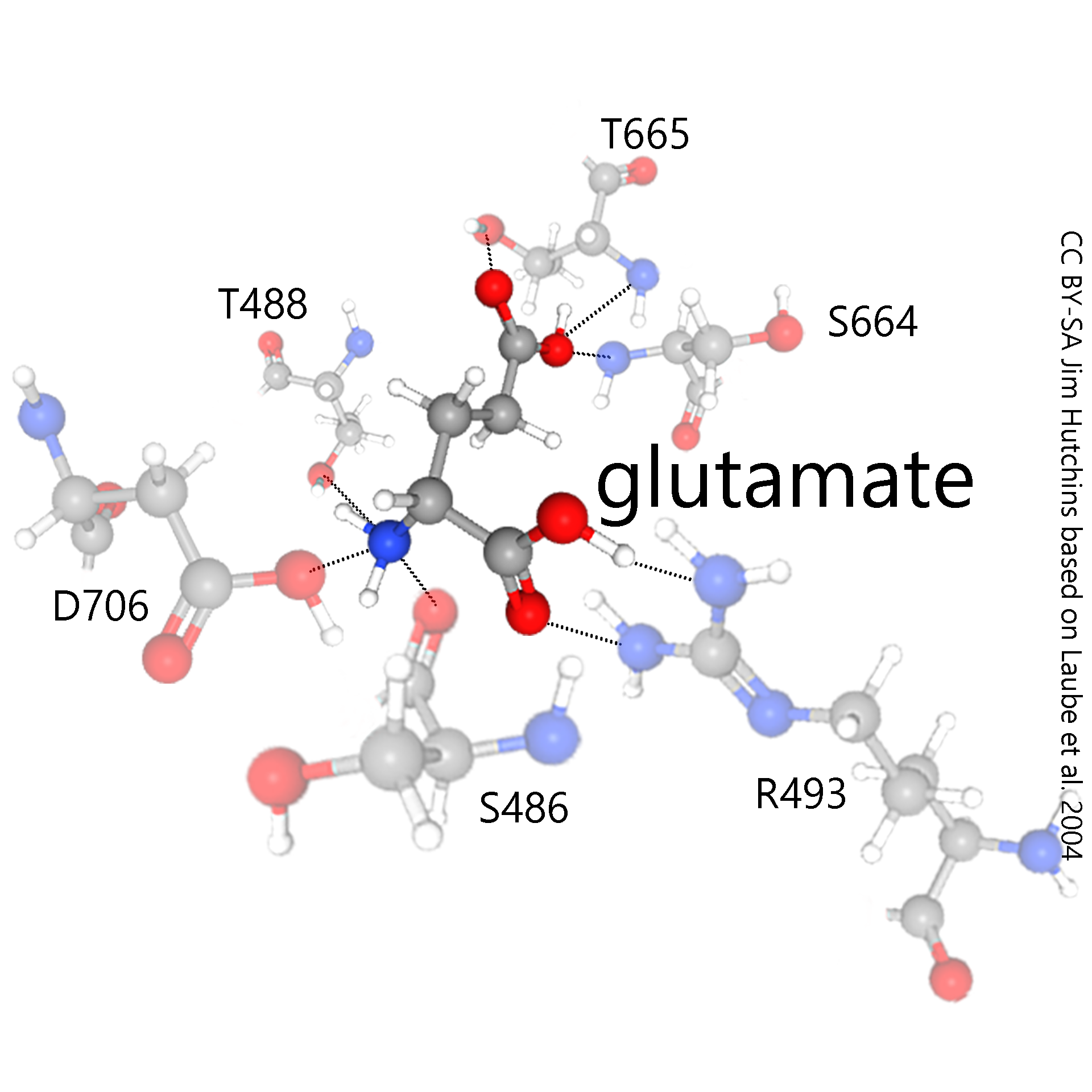 The mechanism of glutamate binding to the NMDA receptor is a good example of how neurotransmitter receptors work. The NMDA receptor is a protein which is, like all proteins, formed from a string of amino acids joined by strong bonds called peptide bonds. Each amino acid can be indicated by a single letter code. Then, a number following the letter indicates its position in the amino acid chain. These numbers are assigned sequentially, in the order that amino acids are added by tRNA at the ribosome. When a protein becomes folded, the folding process does not necessarily respect this sequential order.
The mechanism of glutamate binding to the NMDA receptor is a good example of how neurotransmitter receptors work. The NMDA receptor is a protein which is, like all proteins, formed from a string of amino acids joined by strong bonds called peptide bonds. Each amino acid can be indicated by a single letter code. Then, a number following the letter indicates its position in the amino acid chain. These numbers are assigned sequentially, in the order that amino acids are added by tRNA at the ribosome. When a protein becomes folded, the folding process does not necessarily respect this sequential order.
In this example, serine-486 (S486), threonine-488 (T488), arginine-493 (R493), serine-664 (S664), threonine-655 (T655), and aspartate-706 (D706) all come together to form a pocket which can bind glutamate and no other substance.
This pocket is lined with hydrophilic amino acid residues. These residues are hydrophilic because they are able to form hydrogen bonds with water and other hydrophilic compounds, like glutamate.
Hydrogen bonds are shown as dotted lines in the illustrations, indicating their transient and half-hearted nature.
Within the lining of the pocket, the pattern of hydrogen bonding in the amino acid residues exactly matches the hydrogen bonding properties of the glutamate molecule. For example, wherever the glutamate molecule has a negatively-charged, hydrogen-donating carboxylic acid (—COOH) group, the receptor protein provides one or more positively-charged, hydrogen-accepting amino (—NH2) groups. When the carboxylic acid group donates its hydrogen, or partially donates it, it becomes —COO–. When the amino group accepts a hydrogen, or partially accepts it, it becomes —NH3+. The partial, or transient, attraction between these partial, or transient, opposite charges is the essence of hydrogen bonding. In this way, glutamate is held in the NMDA receptor pocket and the NMDA receptor changes shape to allow the passage of Na+ or Na+ and Ca2+ ions.
Why Doesn’t Glutamine Bind to the NMDA Receptor?
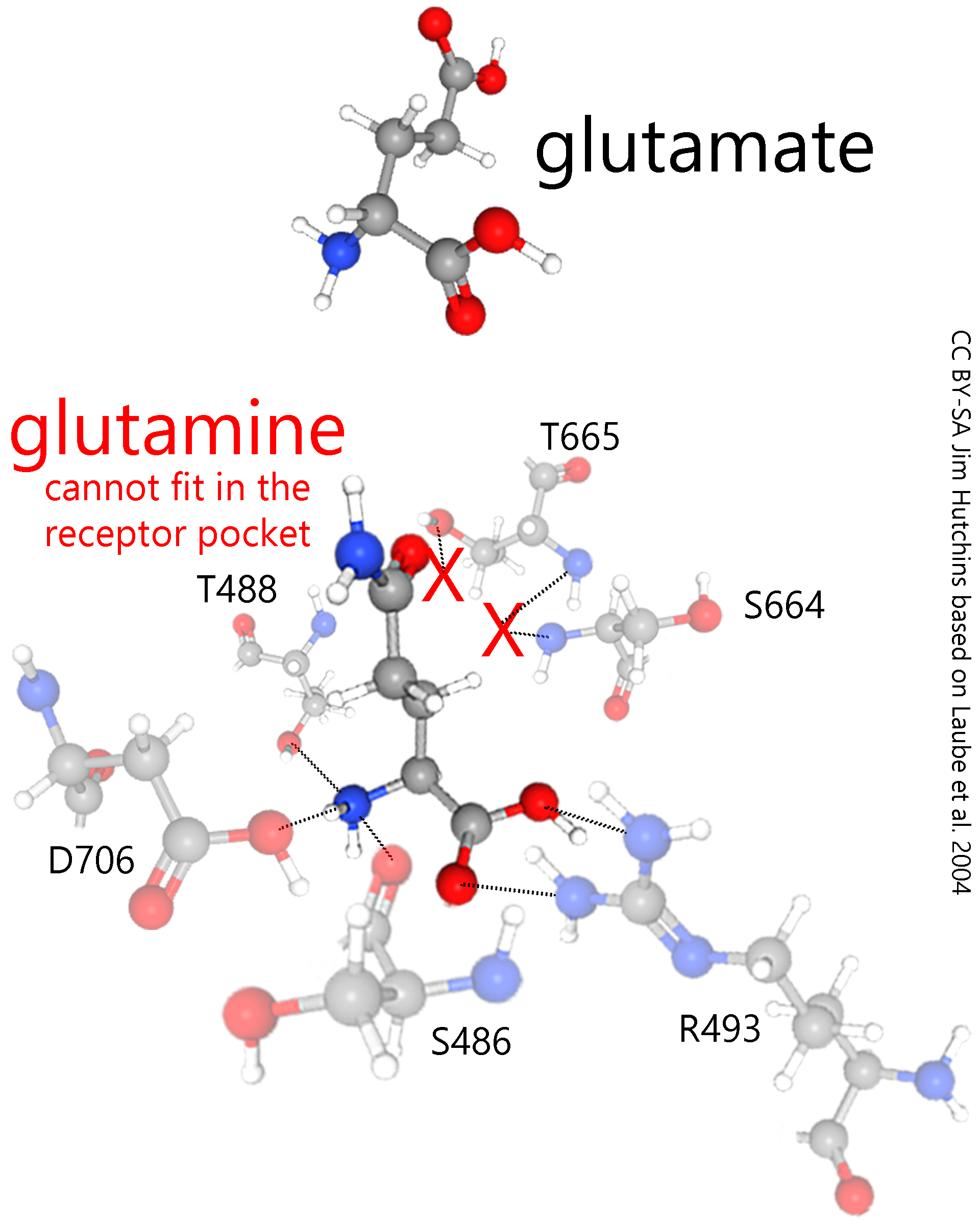
By simpling removing a hydroxyl (–OH) group in the carboxylic acid (–COOH) group, and replacing it with an amide (–CONH2) group, glutamate becomes glutamine and completely changes its hydrogen bonding properties.
Now it no longer fits within the NMDA receptor pocket (red Xs). Since it cannot bind to the receptor, it cannot trigger toxic levels of Ca2+ entry and can be passed harmlessly through the extracellular space.
Metabotropic Glutamate Receptors
The metabotropic glutamate receptors all act through some sort of G protein. When glutamate binds to its receptor, the receptor changes shape and releases a G protein which slides in the plane of the neuronal membrane.
Metabotropic glutamate receptors come in eight types (mGluR1 through mGluR8) that are divided up into three groups.
Group I metabotropic glutamate receptors (mGluR1 and mGluR5) are mostly found in the postsynaptic membrane. The receptor releases a G protein in the class called Gq. That is, they act through a inositol triphosphate-diacylglycerol-calcium second messenger system. Because they ultimately result in Ca2+ release, group I mGluRs potentiate the response to glutamate at NMDA receptors.
Group II metabotropic glutamate receptors (mGluR4, mGluR6, mGluR7 and mGluR8)
Group III metabotropic glutamate receptors (mGluR1 and mGluR5)
Glutamate Removal and Recycling
Media Attributions
- Management of Glutamate at the Synapse © Jim Hutchins, Lindsay Aune, and Mina Nashed | BioRender is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- NMDA Receptor Acts as a Coincidence Detector © Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license

