Neurotransmitters: Gases
Jackson Stringham and Jim Hutchins
Chapter under construction. This is the first draft. If you have questions, or want to help in the writing or editing process, please contact hutchins.jim@gmail.com.
Neuronal communication extends beyond classical neurotransmitters to include gaseous signaling molecules such as nitric oxide (NO), carbon monoxide (CO), and hydrogen sulfide (H₂S). Unlike conventional neurotransmitters, these gases are not stored in vesicles but are synthesized on demand, diffuse freely across membranes, and exert localized or retrograde effects on synaptic activity. This chapter details their synthesis, signaling mechanisms, and functional roles within the nervous system.
Nitric Oxide (NO)
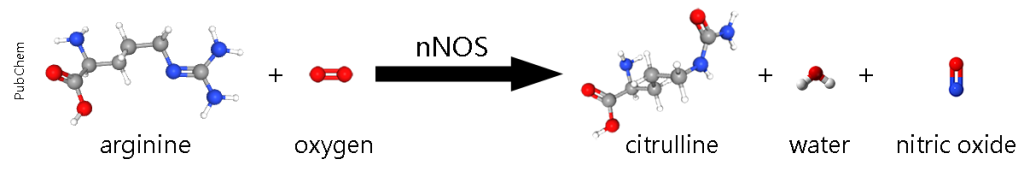
Nitric oxide is synthesized from the amino acid L-arginine through the enzymatic action of nitric oxide synthase (NOS). Three isoforms of NOS exist: neuronal NOS (nNOS/NOS-1), constitutively expressed in neurons and activated by Ca²⁺/calmodulin following NMDA receptor stimulation by glutamate; endothelial NOS (eNOS), which is constitutively active in blood vessels; and inducible NOS (iNOS), expressed in immune cells during inflammation. The synthesis of NO requires molecular oxygen, NADPH, and cofactors such as tetrahydrobiopterin and flavin adenine dinucleotide (Fig. #). Once produced, NO is rapidly inactivated by reactions with hemoglobin or iron-containing compounds.
NO primarily acts by binding to soluble guanylyl cyclase (sGC), stimulating the production of cyclic guanosine monophosphate (cGMP). Elevated cGMP activates protein kinases that phosphorylate target proteins, mediating effects such as vasodilation and synaptic plasticity. A secondary mechanism involves S-nitrosylation, where NO modifies cysteine residues on ion channels and enzymes, altering their activity. Notably, NO also serves as a retrograde messenger in processes like depolarization-induced suppression of inhibition (DSI), where postsynaptic NO release transiently suppresses GABAergic transmission in the hippocampus and cerebellum. Functionally, NO enhances synaptic plasticity, supports neuroprotection by improving blood flow and mitochondrial function, and contributes to excitotoxicity at excessive levels. Its synthesis is tightly linked to NMDA receptor activity through interactions with the postsynaptic protein PSD95, ensuring precise regulation during glutamatergic signaling.
Carbon Monoxide (CO)
Carbon monoxide is generated by the enzyme heme oxygenase (HO), which breaks down heme into biliverdin, iron, and CO. The constitutive isoform HO2 is highly expressed in neurons of the cerebellum, hippocampus, and olfactory bulb, while HO1 is inducible in glial cells under stress. CO signaling parallels that of NO, as it activates soluble guanylyl cyclase (sGC) to elevate cGMP levels. However, HO2 and sGC exhibit near-identical localization in neurons, enabling precise modulation of cGMP-dependent pathways.
In the olfactory system, CO regulates cyclic nucleotide-gated channels via cGMP, mediating sensory adaptation to odorants. Within the enteric nervous system, CO contributes to non-adrenergic, non-cholinergic transmission, a role confirmed by genetic studies showing that mice lacking both HO2 and nNOS lose this signaling entirely. Despite its physiological roles, research on CO remains limited by the lack of selective HO2 inhibitors.
Hydrogen Sulfide (H₂S)
Hydrogen sulfide is synthesized by cystathionine-β-synthase (CBS), an enzyme that converts L-cysteine to H₂S. CBS activity is enhanced by Ca²⁺/calmodulin following Ca²⁺ influx through NMDA or AMPA receptors, as well as by glutamate signaling (Fig. #). H₂S modulates neuronal activity by increasing cAMP levels through adenylate cyclase activation and by opening ATP-sensitive K⁺ channels, leading to membrane hyperpolarization.
Exogenous H₂S enhances hippocampal long-term potentiation (LTP), though mice genetically lacking CBS retain normal LTP, suggesting redundant mechanisms. H₂S also supports neuroprotection by maintaining redox balance and mitochondrial function. Its dual roles- as a facilitator of plasticity and a guardian against oxidative stress- highlight its complexity as a gaseous neurotransmitter.
Unique Features of Gaseous Neurotransmitters
Gaseous neurotransmitters differ fundamentally from classical neurotransmitters. They are not stored but synthesized rapidly in response to stimuli, diffuse freely across membranes without requiring receptors, and act locally or retrogradely. Their effects are transient due to rapid inactivation: NO reacts with hemoglobin, CO is metabolized, and H₂S is oxidized. All three gases exhibit dose-dependent duality, exerting neuroprotective effects at low levels but contributing to toxicity at high concentrations.
Conclusion
Nitric oxide, carbon monoxide, and hydrogen sulfide represent a unique class of neurotransmitters that integrate synaptic activity with metabolic and homeostatic processes. Through cGMP/cAMP pathways, ion channel modulation, and retrograde signaling, they fine-tune neural circuits in ways distinct from traditional chemical transmission. Their roles in synaptic plasticity, sensory adaptation, and neuroprotection underscore their significance in both physiological and pathological contexts. Future research, particularly into selective inhibitors and genetic models, will further elucidate their contributions to brain function and disease.

