Neurotransmitters: Acetylcholine
Jim Hutchins
Chapter under construction. This is the first draft. If you have questions, or want to help in the writing or editing process, please contact hutchins.jim@gmail.com.
The Cholinergic Synapse
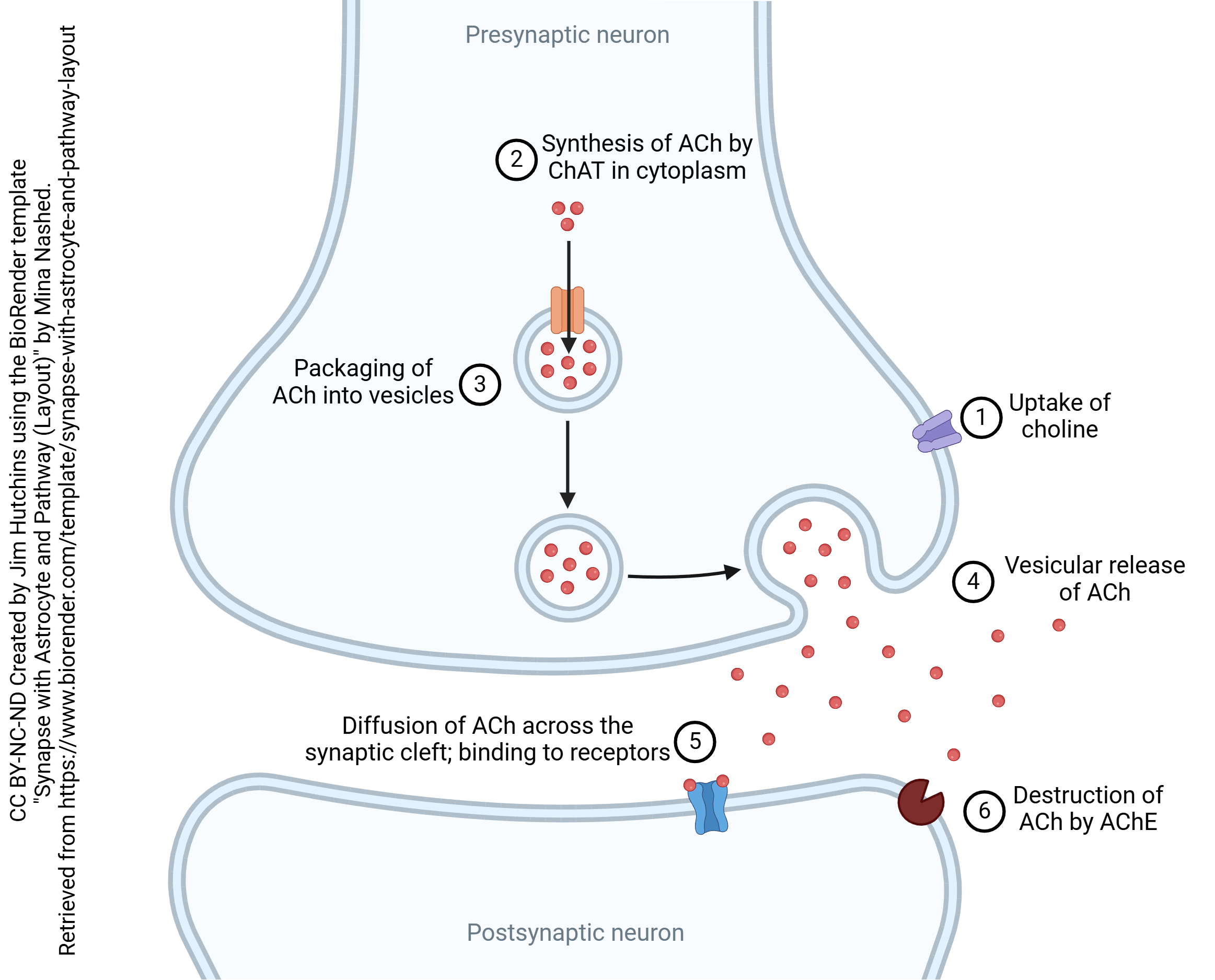 Much of what we know about chemical synapses was first discovered when scientists studied the cholinergic synapse. Acetylcholine (ACh) was first synthesized in 1867. In 1906, Hunt and Taveau showed that extremely dilute preparations of ACh stop the heart and lungs. Sir Henry Dale, working in the Wellcome pharmaceutical labs, showed that a mushroom extract (muscarine) and a tobacco extract (nicotine) acted on nerve cells, but that ACh was even stronger in evoking the same effects. Otto Loewi’s mysterious and powerful Vagusstoff, discovered in 1921, was then shown to be ACh. Over the next 40 years, a leech muscle preparation treated with a compound (eserine) which inhibited the destruction of ACh was the standard technique for measuring the action of ACh and related compounds. The neuromuscular junction, where ACh is released onto muscle cells, was extensively studied by physiologists; the quantal release of neurotransmitter was extensively characterized at this synapse. Because of a quirk of biology (described below), scientists were able to isolate the ACh receptor protein in large quantities before the biochemical properties of any other neurotransmitter receptor were well-characterized. Indigenous peoples have used drugs which alter the function of the cholinergic synapse for millenia, and European military scientists discovered nerve agents which also affect synaptic transmission at the cholinergic synapse.
Much of what we know about chemical synapses was first discovered when scientists studied the cholinergic synapse. Acetylcholine (ACh) was first synthesized in 1867. In 1906, Hunt and Taveau showed that extremely dilute preparations of ACh stop the heart and lungs. Sir Henry Dale, working in the Wellcome pharmaceutical labs, showed that a mushroom extract (muscarine) and a tobacco extract (nicotine) acted on nerve cells, but that ACh was even stronger in evoking the same effects. Otto Loewi’s mysterious and powerful Vagusstoff, discovered in 1921, was then shown to be ACh. Over the next 40 years, a leech muscle preparation treated with a compound (eserine) which inhibited the destruction of ACh was the standard technique for measuring the action of ACh and related compounds. The neuromuscular junction, where ACh is released onto muscle cells, was extensively studied by physiologists; the quantal release of neurotransmitter was extensively characterized at this synapse. Because of a quirk of biology (described below), scientists were able to isolate the ACh receptor protein in large quantities before the biochemical properties of any other neurotransmitter receptor were well-characterized. Indigenous peoples have used drugs which alter the function of the cholinergic synapse for millenia, and European military scientists discovered nerve agents which also affect synaptic transmission at the cholinergic synapse.
Choline Uptake is the Rate-Limiting Step in Acetylcholine Synthesis
The first step in the production and release of acetylcholine is choline uptake by the high-affinity choline transporter, also known as solute carrier family 5 member 7, or by its gene name, SLC5A7. Choline is in short supply in the body and this is the rate-limiting step in acetylcholine synthesis and release.
Synthesis of Acetylcholine
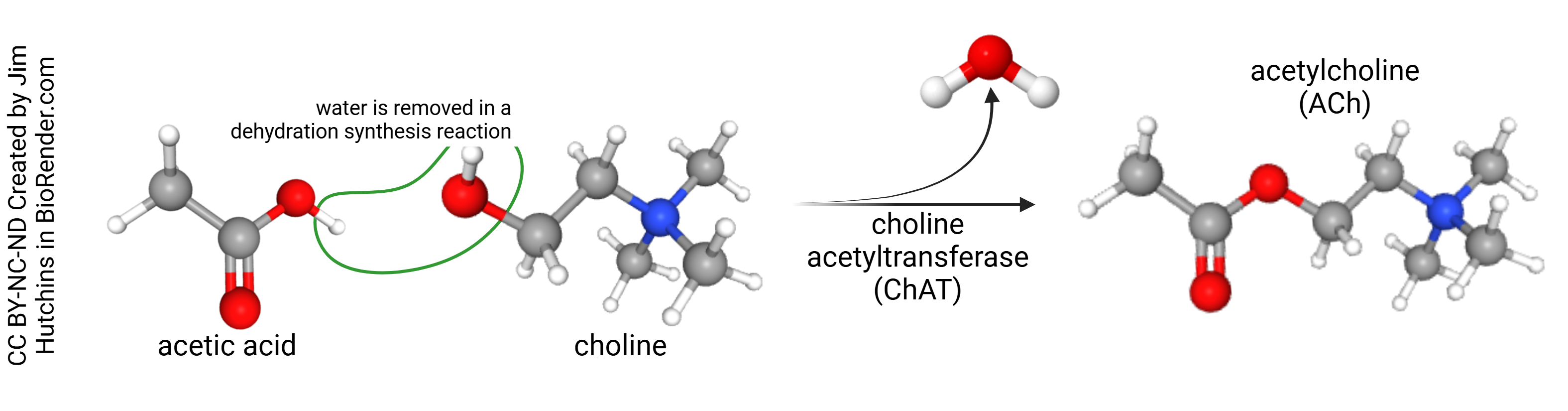 Acetic acid exists mostly as acetate ion (acetic acid minus a hydrogen ion, H+) at physiological pH. This combines with the nitrogen-containing compound choline to form acetylcholine with the removal of a water molecule (i.e. a dehydration synthesis reaction). This reaction is catalyzed by the enzyme choline acetyltransferase (ChAT).
Acetic acid exists mostly as acetate ion (acetic acid minus a hydrogen ion, H+) at physiological pH. This combines with the nitrogen-containing compound choline to form acetylcholine with the removal of a water molecule (i.e. a dehydration synthesis reaction). This reaction is catalyzed by the enzyme choline acetyltransferase (ChAT).
Packaging of Acetylcholine into Vesicles
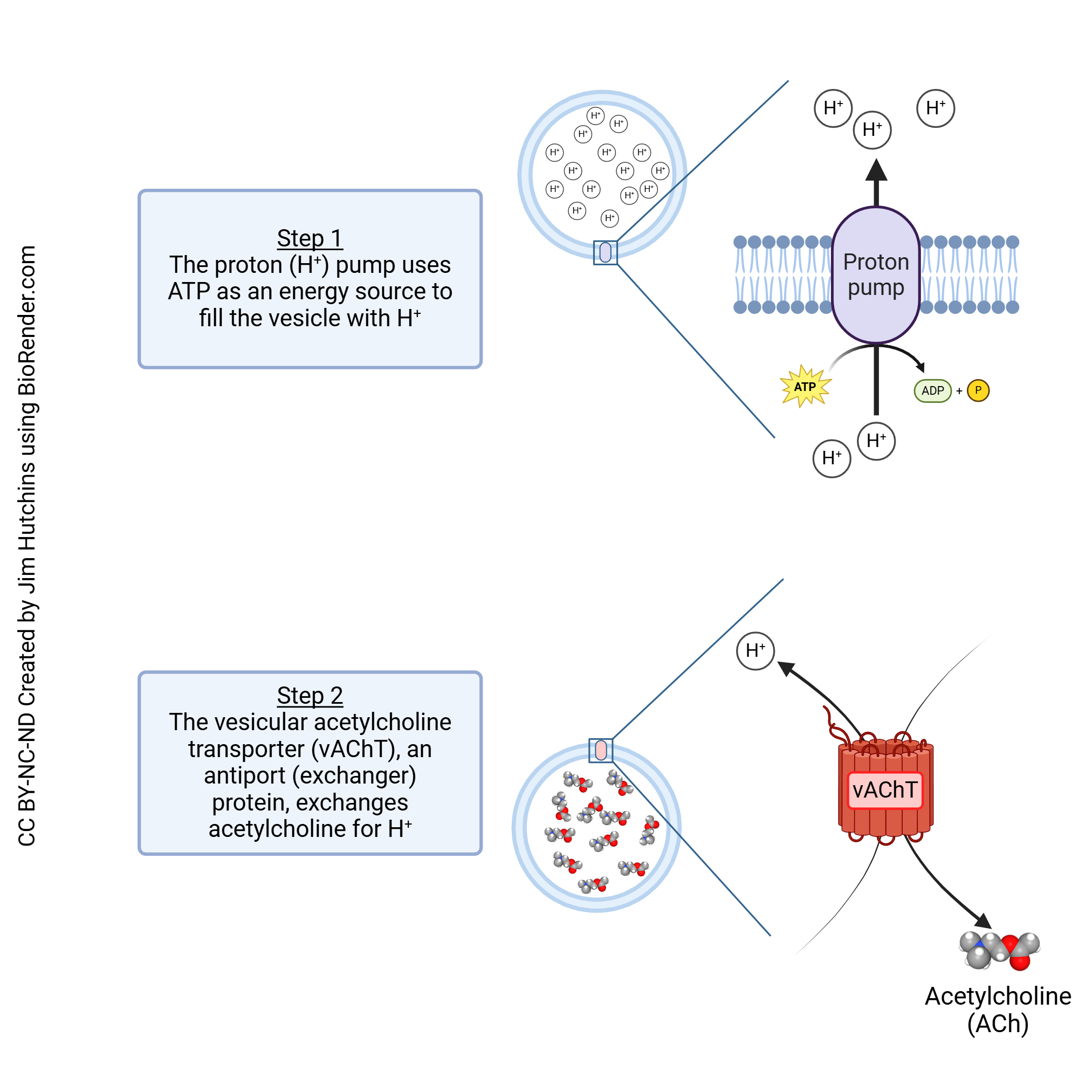
Like other neurotransmitters, the packaging of acetylcholine into vesicles is a two-step process.
First, a proton pump which concentrates protons against their concentration gradient is used to reduce the pH inside the vesicle. This requires energy in the form of ATP. Now the vesicle has a much higher concentration of protons than the surrounding cytoplasm.
In the next step, the protons are used to drive an antiport (exchanger) protein, the vesicular acetylcholine transporter (vAChT). This transport protein uses the hydrogen ion gradient to drive the movement of acetylcholine into the vesicle as it allows H+ to flow down its concentration gradient and into the cytoplasm.
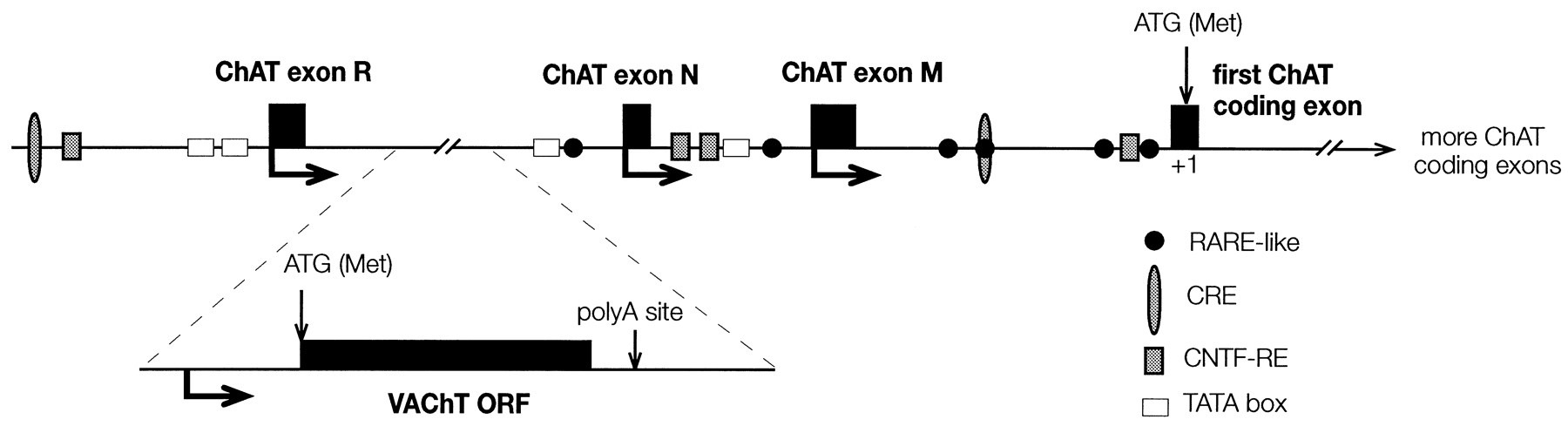
The vAChT is member 3 of solute carrier family 18, so the gene is designated SLC18A3. The SLC18A3 gene is located within the first intron of the choline acetyltransferase gene. In other words, the regulatory regions of both the ChAT and vAChT genes are bound by the same transcription factors, so they are turned on or off at the same time. The vAChT gene, SLC18A3, is cleaved out of the pre-mRNA for ChAT by the spliceosome and then becomes the mRNA for vAChT.
Release of Acetylcholine
The neuromuscular junction is a cholinergic synapse that has been used as a model system for the chemical synapse for the last century. Thus, the vesicular release of acetylcholine has been covered in other chapters covering the canonical chemical synapse and quantal release of neurotransmitter.
Binding of Acetylcholine to Receptors
Two Different Pharmacological Types of Acetylcholine Receptor Types Were Found
Dale and other physiologists of the early 20th century systematically characterized the pharmacology of the cholinergic synapse using a variety of naturally-occurring and synthesized compounds. Even before the concept of a neurotransmitter receptor had been formulated, these studies identified two categories of receptor:
- those that respond to ACh but only to the agonist nicotine;
- those that respond to ACh but only to muscarine as an agonist.
Thus, Dale divided the pharmacological response to ACh into a nicotinic and a muscarinic response.
We now know that these responses are mediated by nicotinic ACh receptors and muscarinic ACh receptors, respectively.
Nicotinic acetylcholine receptors were found at the neuromuscular junction and the preganglionic (i.e. first) synapses of the sympathetic nervous system and parasympathetic nervous system.
Muscarinic acetylcholine receptors were found at the postganglionic (i.e. second) synapses of the parasympathetic nervous system (e.g. in the iris sphincter muscle; in the pacemaker cells of the heart) and in a variety of brain locations.
Nicotinic Acetylcholine Receptors
 The drug nicotine, extracted from tobacco leaves, is an agonist at the receptors which came to be called nicotinic. Nicotine increases the activity of the neuromuscular junction and is therefore an agonist there.
The drug nicotine, extracted from tobacco leaves, is an agonist at the receptors which came to be called nicotinic. Nicotine increases the activity of the neuromuscular junction and is therefore an agonist there.
 It has been known since antiquity that some species of fish, popularly known as electric eels, can produce large electric shocks that can kill or incapacitate divers. (According to legend, Alessandro Volta was inspired to invent the battery based on the properties of these fish, who carried biological batteries.) These electric currents are generated in an organ called the electroplax. For example, each cell in the electroplax of Torpedo californica (pictured here) can produce 0.15 volts (V), or about 1/10 of the voltage generated by a AA battery. The electroplax contains about 1000 such cells, for a total output of 150 V. About 3000 electrons pass through each nicotinic acetylcholine receptor (nAChR) during the 1 msec it is open. There are 50,000,000,000 (5 x 1010) nAChRs in the Torpedo electroplax. This is such a rich source of protein that the nAChR was the first postsynaptic receptor protein purified by scientists.
It has been known since antiquity that some species of fish, popularly known as electric eels, can produce large electric shocks that can kill or incapacitate divers. (According to legend, Alessandro Volta was inspired to invent the battery based on the properties of these fish, who carried biological batteries.) These electric currents are generated in an organ called the electroplax. For example, each cell in the electroplax of Torpedo californica (pictured here) can produce 0.15 volts (V), or about 1/10 of the voltage generated by a AA battery. The electroplax contains about 1000 such cells, for a total output of 150 V. About 3000 electrons pass through each nicotinic acetylcholine receptor (nAChR) during the 1 msec it is open. There are 50,000,000,000 (5 x 1010) nAChRs in the Torpedo electroplax. This is such a rich source of protein that the nAChR was the first postsynaptic receptor protein purified by scientists.
Once this pure source of nAChRs was discovered, scientists could characterize the structural properties of the nAChR. The receptor is a pentamer; it consists of five protein chains. The receptors found in adult skeletal muscle and the electroplax consist of 2 α1 receptors and 1 each of the β1, δ, and ε subunits. At the embryonic neuromuscular junction, nAChR have another protein product (designated γ) and are missing the ε subunit in a (α1)2β1γδ configuration.
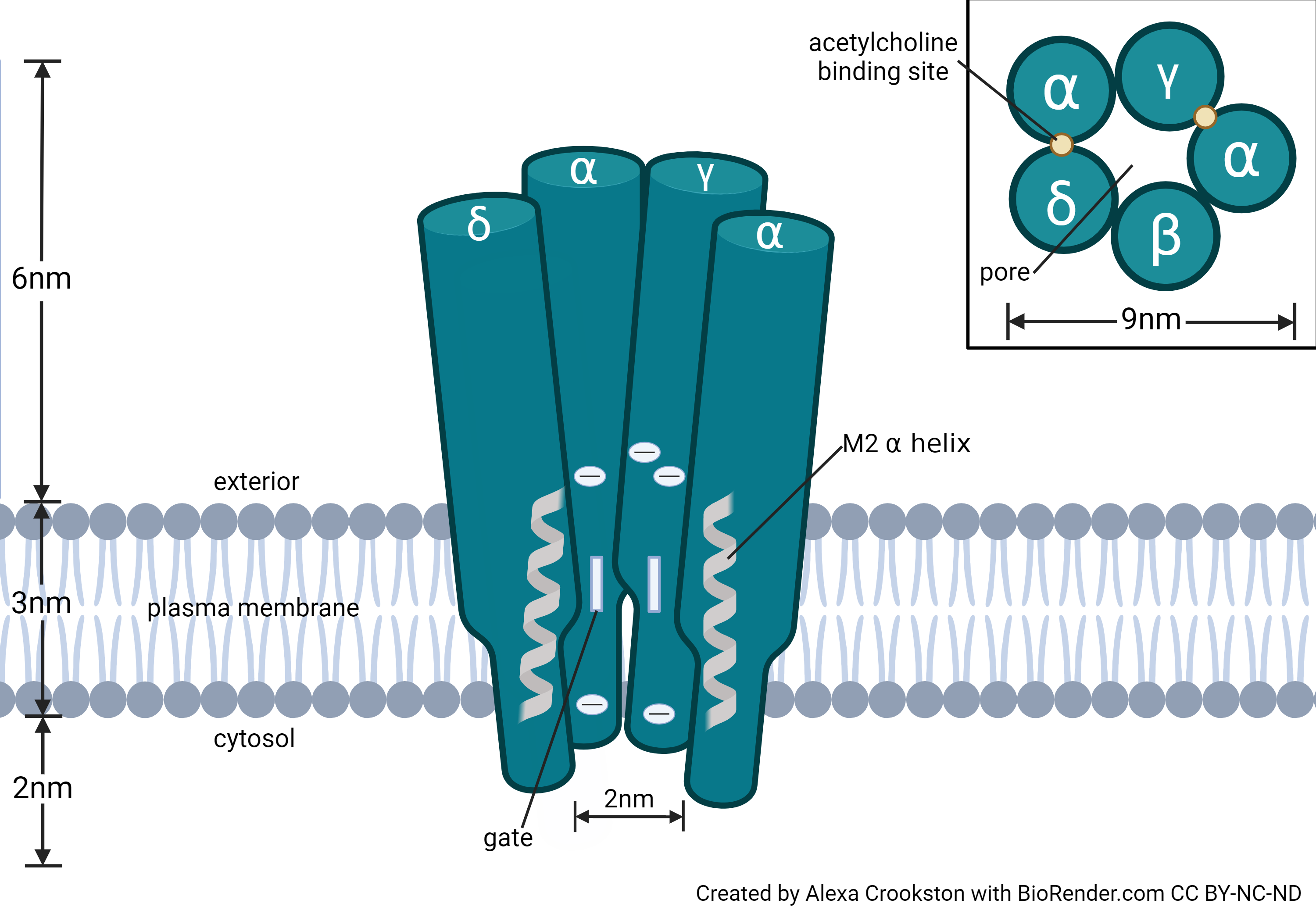 In neurons, there are nine different gene products forming α subunits and four different gene products forming β subunits. These are designated α2−α10 and β2−β4. Examples of neuronal nAChRs include:
In neurons, there are nine different gene products forming α subunits and four different gene products forming β subunits. These are designated α2−α10 and β2−β4. Examples of neuronal nAChRs include:
- (α4)3(β2)2
- (α4)2(β2)3
- (α3)2(β4)3
- α4α6β3(β2)2
- (α7)5
Acetylcholine binds to the α-γ and α-δ interfaces of the acetylcholine receptor. Both ACh binding sites must be occupied for the receptor to change shape and allow the passage of cations, including Na+, K+, and Ca2+.
 A number of indigenous people in the Caribbean basin use a preparation from the vines Chondrodendron tomentosum (curare vine) and Strychnos species to paralyze and incapacitate animals they were hunting. The Mukusi people of modern-day Guyana gave the preparation its name, wurari. Although the name curare comes from Sir Walter Raleigh’s accounts of explorations in Guyana, the first to characterize the pharmacology of curare (in 1780) was the Tuscan father of neurotoxicology, Abbé Felice Fontana.
A number of indigenous people in the Caribbean basin use a preparation from the vines Chondrodendron tomentosum (curare vine) and Strychnos species to paralyze and incapacitate animals they were hunting. The Mukusi people of modern-day Guyana gave the preparation its name, wurari. Although the name curare comes from Sir Walter Raleigh’s accounts of explorations in Guyana, the first to characterize the pharmacology of curare (in 1780) was the Tuscan father of neurotoxicology, Abbé Felice Fontana.
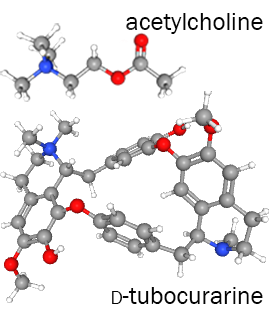 The active ingredient, d-tubocurarine, binds to the α-γ and α-δ interfaces of the acetylcholine receptor and blocks the ACh binding site on the α subunit.
The active ingredient, d-tubocurarine, binds to the α-γ and α-δ interfaces of the acetylcholine receptor and blocks the ACh binding site on the α subunit.
The Strychnos genus is the source of several neurotoxins. For example, the tree Strychnos nux-vomica (i.e. “nut causing vomiting”), native to tropical Asia, is the source of strychnine, a poison used to block glycinergic signaling.
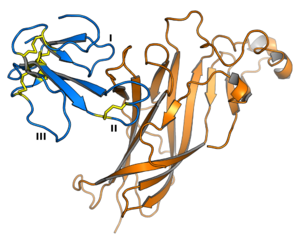
α-bungarotoxin is shown in blue. One loop of the nAChR protein is shown in orange.In 1963, Chang and Lee isolated α-bungarotoxin from a venomous snake native to Taiwan, the Many-Banded Krait (Bungarus multicinctus). This toxin (shown in blue) binds to the α subunit of the nAChR (part of which is shown in orange) and blocks the ion channel. Therefore, α-bungarotoxin acts as an antagonist of the muscular and neuronal nAChR.

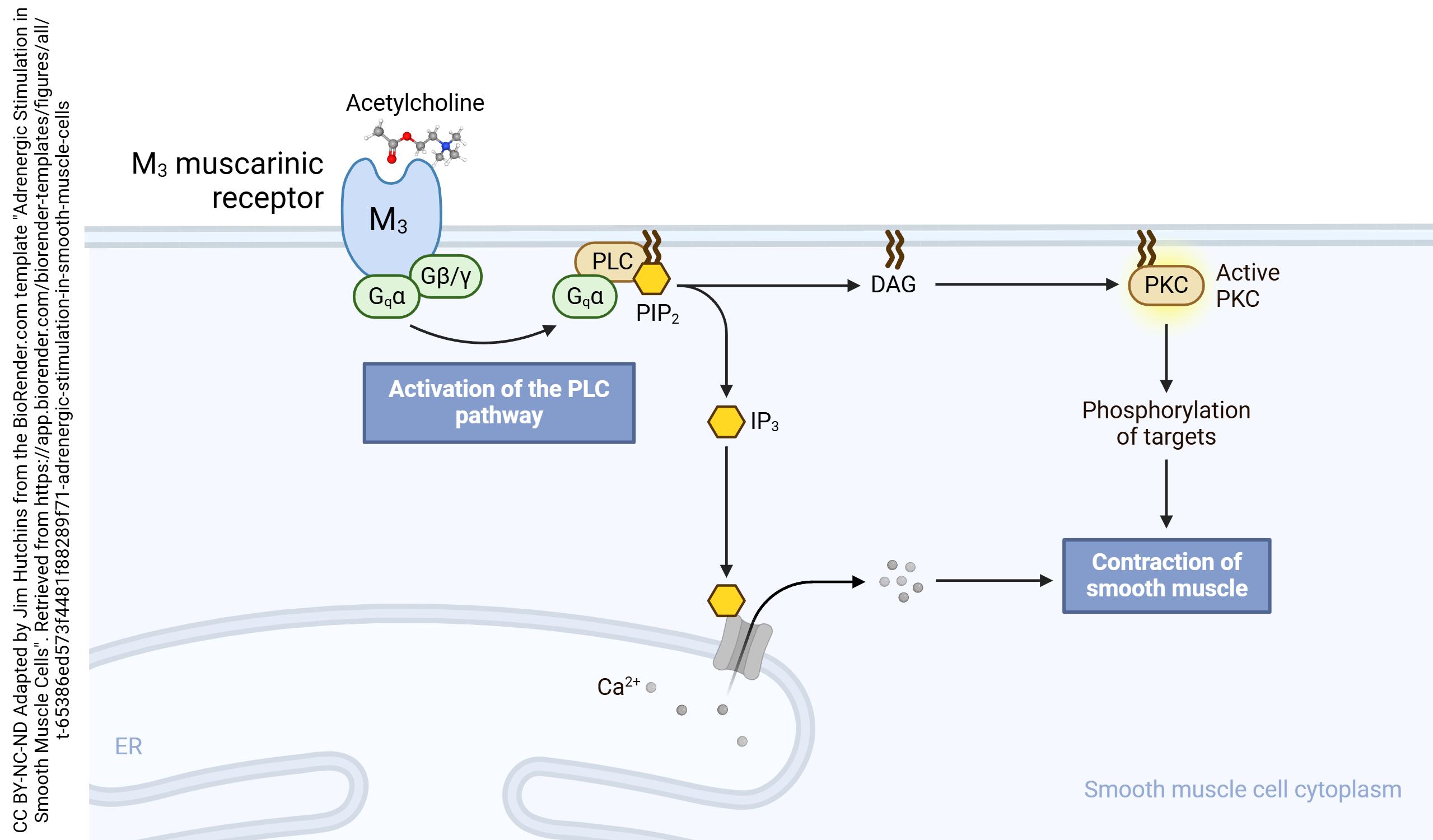
Muscarinic Acetylcholine Receptors

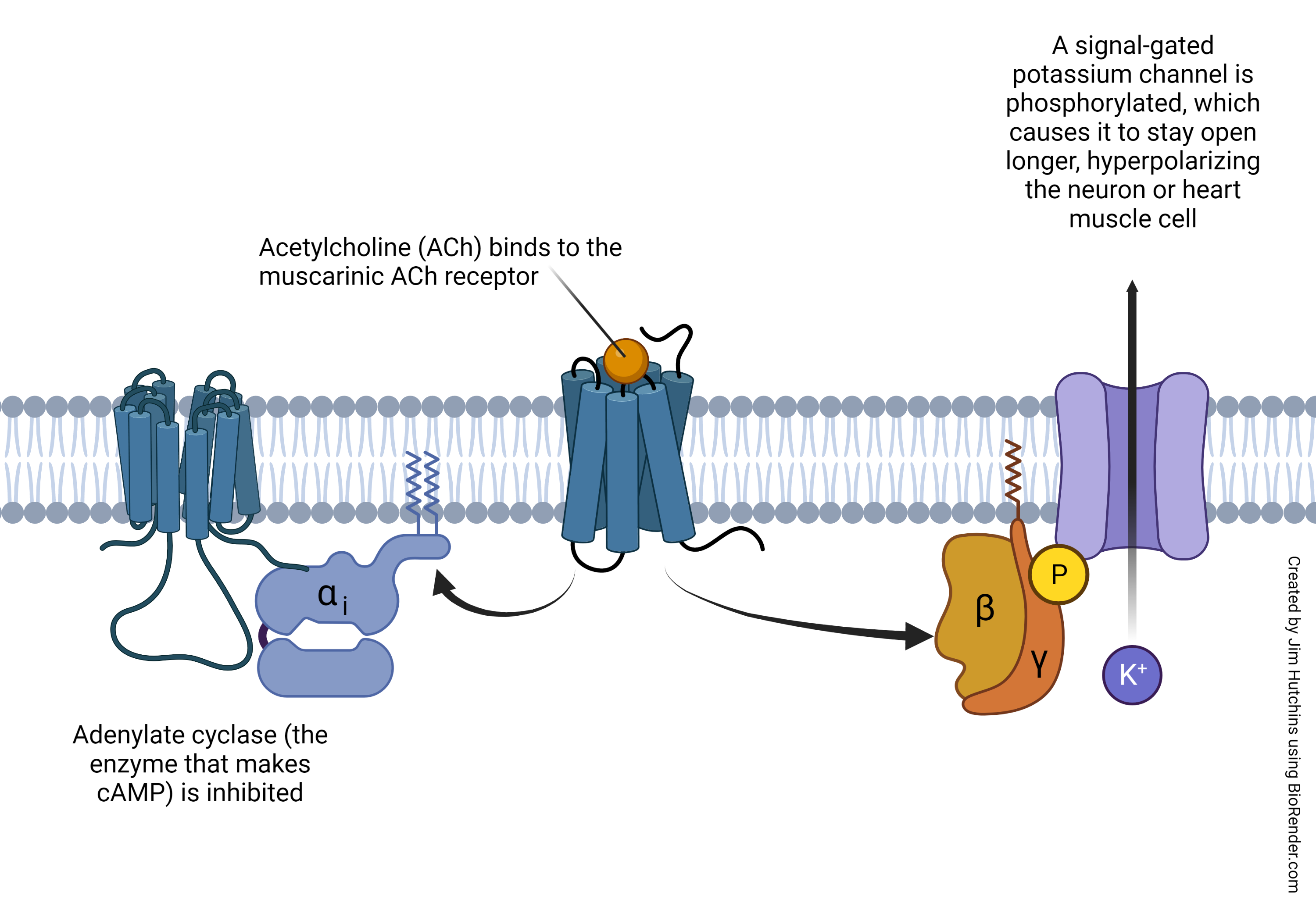
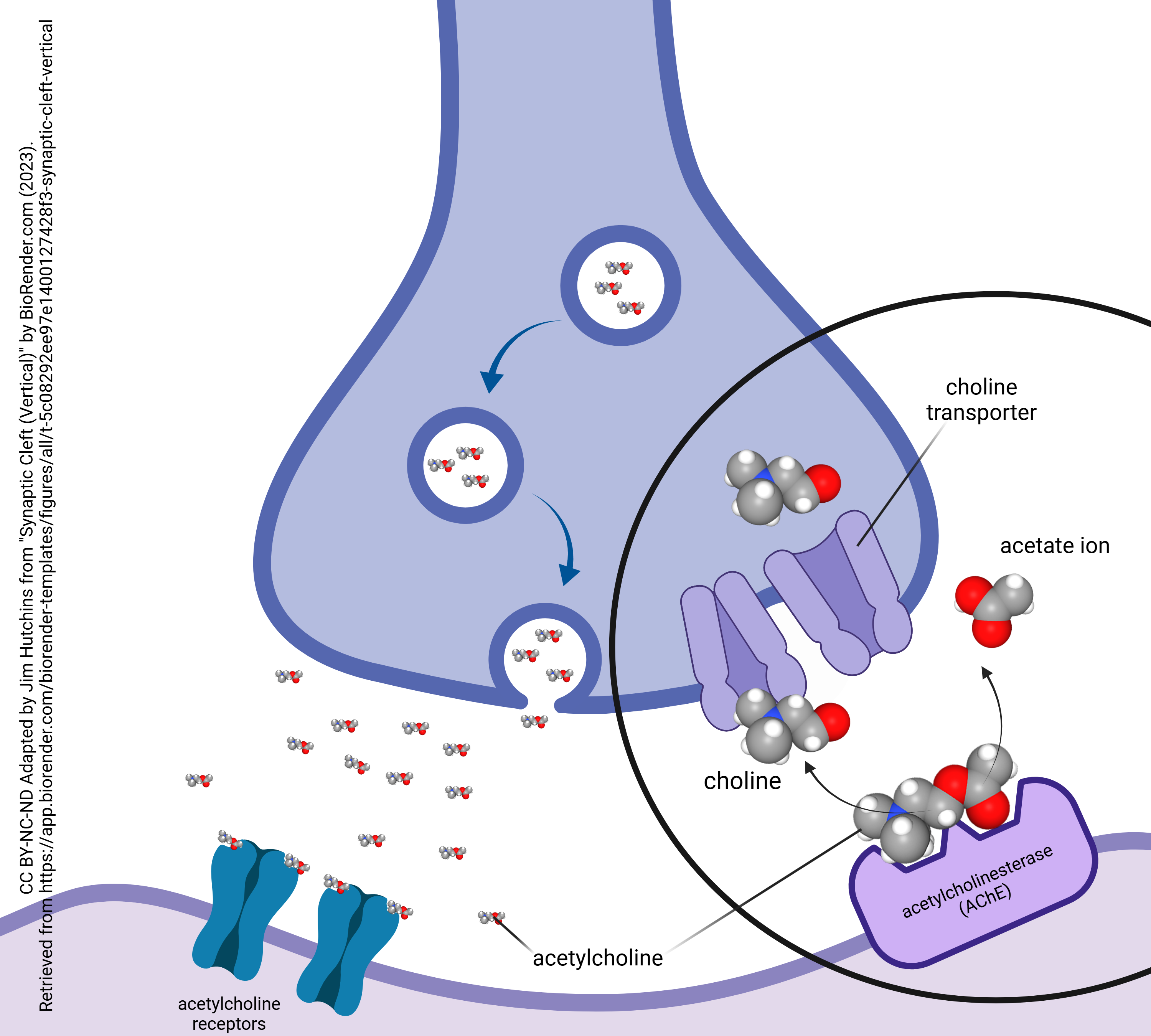
Destruction of Acetylcholine by Acetylcholinesterase
Media Attributions
- The Cholinergic Synapse © Mina Nashed adapted by Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- Synthesis of acetylcholine © Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- Vesicular Acetylcholine Transporter © Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- berse and blusztajn 1995 fig 1 choline acetyltransferase gene © Brygida Berse and Jan Krzysztof Blusztajn is licensed under a CC BY (Attribution) license
- E-Cigarette Broken Cigarette © TBEC Review is licensed under a CC BY (Attribution) license
- Torpedo Californica © Ed Bierman is licensed under a CC BY (Attribution) license
- Nicotinic acetylcholine receptor © Alexa Crookston is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- South American Indians preparing an arrow © Wellcome Collection is licensed under a CC BY (Attribution) license
- Comparison of ach curare © PubChem adapted by Jim Hutchins is licensed under a CC BY-SA (Attribution ShareAlike) license
- Alphabungarotoxin_nachr_4uy2 © Opabinia regalis is licensed under a CC BY-SA (Attribution ShareAlike) license
- Many-banded krait © FearingPredators is licensed under a CC BY-SA (Attribution ShareAlike) license
- Amanita muscaria © Onderwijsgek is licensed under a CC BY-SA (Attribution ShareAlike) license
- Muscarinic acetylcholine receptor © Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- Muscarinic M3 Stimulation in Smooth Muscle Cells © Sally Kim adapted by Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- Acetylcholinesterase at the cholinergic synapse © BioRender adapted by Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license

