Comparing Ionotropic and Metabotropic Receptors
Jim Hutchins
Chapter under construction. This is the first draft. If you have questions, or want to help in the writing or editing process, please contact hutchins.jim@gmail.com.
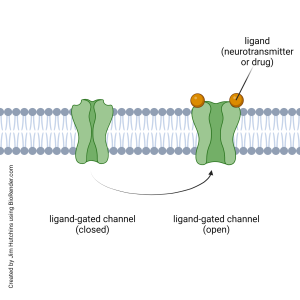
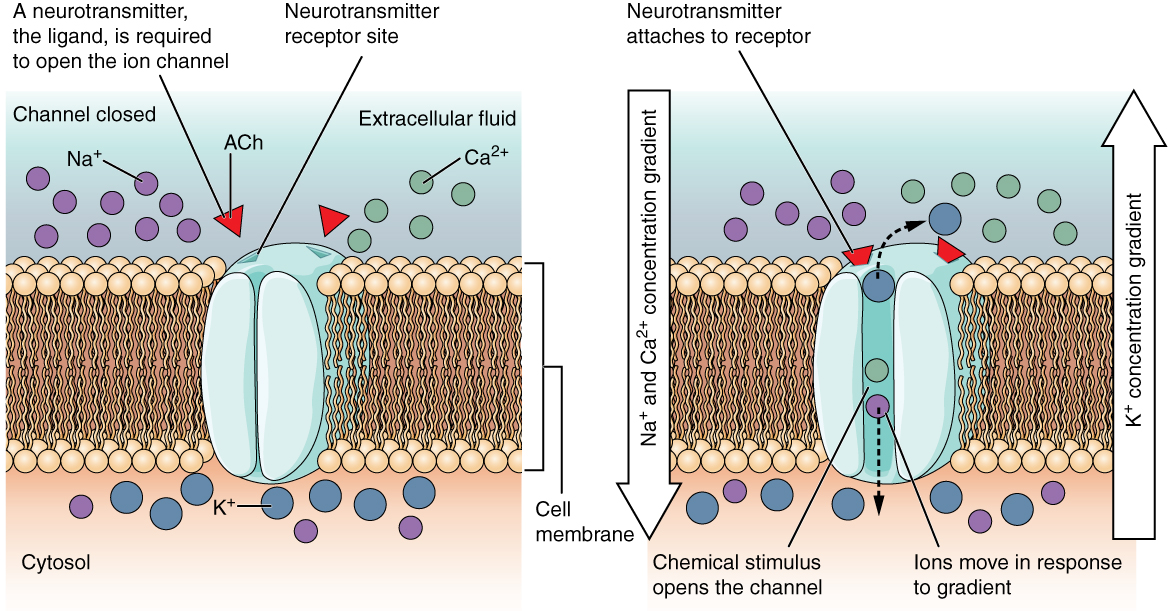
Ionotropic receptors are also called ligand-gated ion channels. When a neurotransmitter, or drug mimicking a neurotransmitter, binds to the ionotropic receptor, it causes the opening of a channel which allows a specific ion (or specific ions) to cross the cell membrane.
The neurotransmitter or drug that binds to the receptor is called a ligand. For example, the nicotinic acetylcholine receptor is an ionotropic receptor. The naturally-occurring ligand is the extracellular signaling molecule acetylcholine.
Among the drugs that bind to the nicotinic acetylcholine receptor are nicotine (the psychoactive ingredient in tobacco or vape juice) and D-tubocurarine. Nicotine is called a receptor agonist because it mimics the “natural” action of acetylcholine: it causes the receptor protein to change shape in the same way that acetylcholine does, opening an ion channel. D-tubocurarine is called a receptor antagonist because it blocks acetylcholine from binding to the receptor and the channel remains closed.
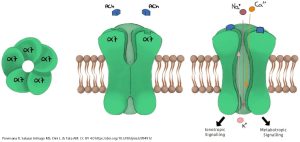
When the ligand binds to the receptor, the receptor protein changes shape from a configuration that does not allow the passage of ions, to one that does.
The nicotinic acetylcholine receptor, when open, allows Na+, K+, and to a lesser extent Ca2+ ions to pass. The five protein subunits that make up the receptor channel form a selective tunnel through which only these three ions can pass. Because the resting membrane potential is well below the equilibrium potentials for Na+ and Ca2+, these ions rush into the neuron through the open channel.
Because the resting membrane potential is above the equilibrium potential for K+, it rushes out of the cell. The net result is a depolarization of the neuron to near 0 mV, generally enough to trigger an action potential or neurotransmitter release. (This value is termed the reversal potential of the receptor.)
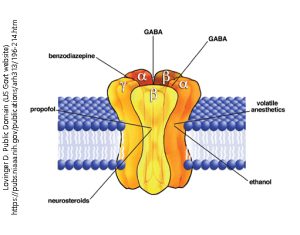
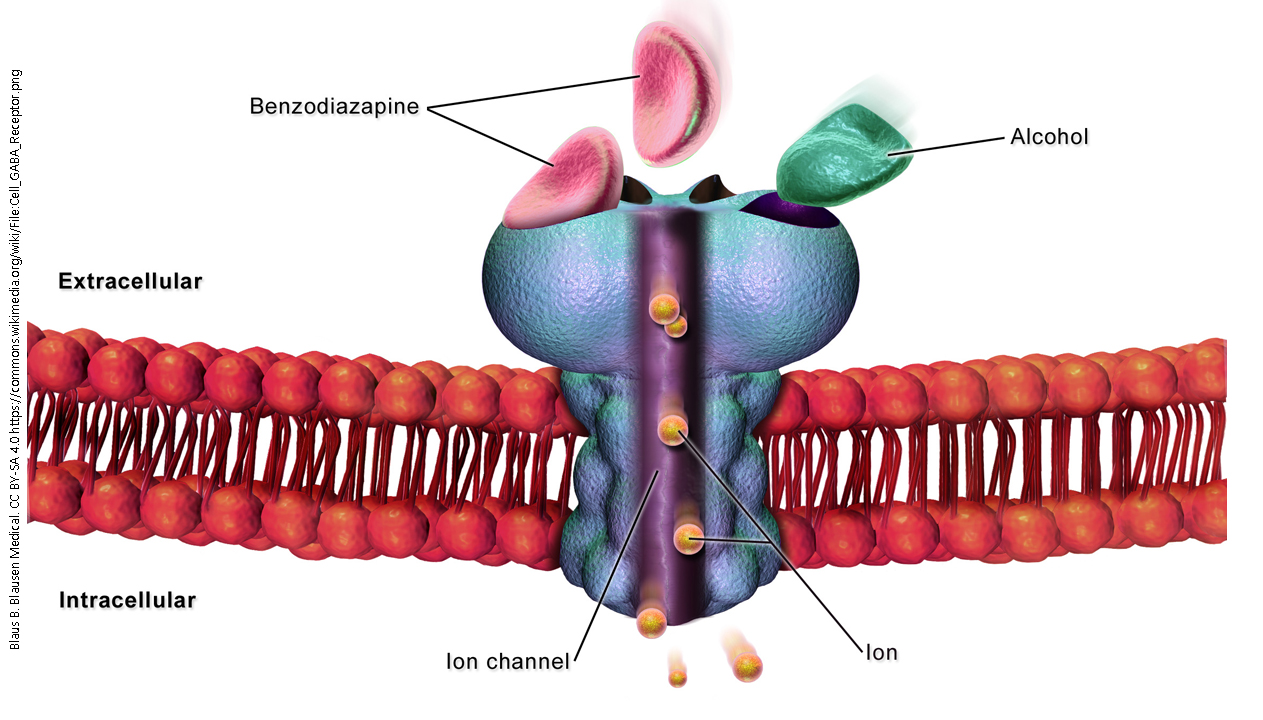
The GABAA receptor is another example of an ionotropic receptor or ligand-gated ion channel. When γ-aminobutyric acid (GABA) binds to the receptor, it allows chloride ions (Cl–) to pass through the ion channel.
Ethanol, propofol, or benzodiazepines (e.g. ValiumTM or XanaxTM) bind to the GABAA receptor, the channel is opened or opened longer. Thus, these are classified as receptor agonists.
Even though ionotropic receptors were the first neurotransmitter receptors studied, and have been known for almost 100 years, subsequent research has shown that there are a limited number of neurotransmitter receptors (fewer than a dozen) that may be classified as ionotropic.
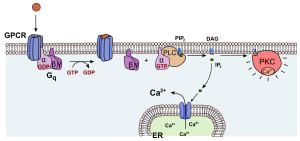
A metabotropic receptor carries this name because it changes the metabolism (biochemistry) of the cell which expresses it. A metabotropic receptors is also called a G protein-coupled receptor (GPCR).
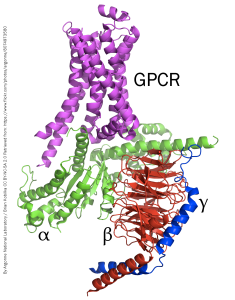
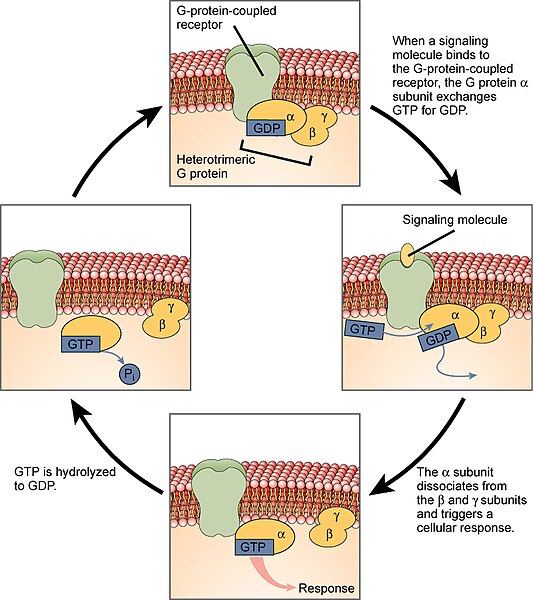
A GPCR has no ion channel. Rather, when a neurotransmitter molecule binds to this type of receptor, the protein changes conformation in such a way that it activates an associated group of three proteins that are collectively called the G protein. The structure of a GPCR and associated G protein is shown at left.
The G protein has three subunits, named in order of their size:
- The α subunit is the largest of the G protein subunits and is most closely associated with the receptor protein.
- The β subunit consists of a “propeller” structure with an array of stacked β-pleated sheets (shown as flat arrows in the diagram).
- The γ subunit is closely associated with the β subunit and they generally operate as a pair.
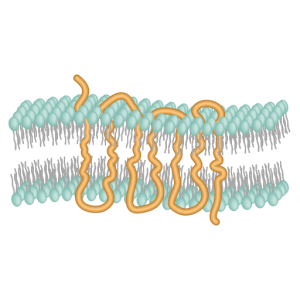
The GPCR itself consists of 7 transmembrane α helices. These keep the GPCR tightly associated with the cell membrane, while the G protein is able to move around in the plane of the membrane on the intracellular side.
When the neurotransmitter binds to the GPCR, its conformation changes. This activates the enzymatic activity of the complex, which phosphorylates the bound guanosine diphosphate (GDP) to now become the triphosphate (GTP). This change to GTP causes the α subunit to change shape, releasing both the α subunit and the βγ pair of subunits. Both pieces of the broken-apart G protein are anchored to the cell membrane, and slide laterally to interact with other proteins.
A typical action for the α subunit is to collide with the enzyme adenylate cyclase. This enzyme, when activated, converts adenosine triphosphate (ATP) to cyclic adenosine monophosphate (cAMP). Cyclic AMP is a ubiquitous and critically-important intracellular signaling molecule.
One type of α subunit, termed αs, stimulates adenylate cyclase activity (note the subscript s denotes stimulation). Another type, termed αi, inhibits adenylate cyclase activity.
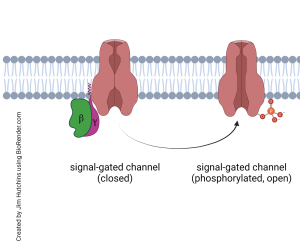
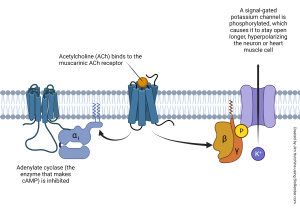
The βγ complex, meanwhile, is also released from the GPCR and also slides in the plane of the membrane, leading to a separate series of actions. Frequently, this means the βγ phosphorylates a signal-gated ion channel, causing it to change shape and either open or close. For example, the βγ associated with the muscarinic acetylcholine receptor (a GPCR) phosphorylates a K+ channel, causing it to open longer. This allows K+ to rush out of the cell, hyperpolarizing it. In this way, GPCRs often cause a change in membrane potential while only indirectly opening or closing an ion channel. This allows for exquisite control of the membrane potential, along with fine control over the levels of intracellular signaling molecules like cAMP.
Media Attributions
- Generic ligand gated channel function © Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- Nicotinic acetylcholine receptor alpha7 © Piovesana, Roberta, Michael Sebastian Salazar Intriago, Luciana Dini, and Ada Maria Tata is licensed under a CC BY (Attribution) license
- GABA receptor © David M. Lovinger is licensed under a Public Domain license
- G protein coupled receptor and g protein structure © Argonne National Laboratory is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- G protein-coupled receptor © DataBase Center for Life Science (DBCLS) is licensed under a CC BY (Attribution) license
- Muscarinic acetylcholine receptor © Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- Generic signal-gated channel function © Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- Heterotrimeric G proteins © Molnar, Charles and Gair, Jane is licensed under a CC BY-SA (Attribution ShareAlike) license
- Activation protein kinase C © Yikrazuul is licensed under a CC BY-SA (Attribution ShareAlike) license
- Ligand-gated Channels © Betts, J. Gordon; Young, Kelly A.; Wise, James A.; Johnson, Eddie; Poe, Brandon; Kruse, Dean H. Korol, Oksana; Johnson, Jody E.; Womble, Mark & DeSaix, Peter is licensed under a CC BY (Attribution) license
- GABA-A receptor © BruceBlaus is licensed under a Public Domain license

