Diffusion and Fick’s First Law
Macady Lunt and Jim Hutchins
Chapter under construction. This is the first draft. If you have questions, or want to help in the writing or editing process, please contact hutchins.jim@gmail.com.
Fick’s First Law
Fick’s first law of diffusion states that substances from an area of higher concentration should move to an area of lower concentration.
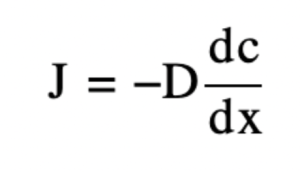
- The letter J is a representation of the flux, which is the number of particles moving past a given region divided by the area of that region multiplied by the time interval.
- The units of J are m-2 s-1
- The letter D represents the diffusion coefficient with units m^2 s.
- The letter C is a representation of the concentration of the gradient with units molecules m-3 (Dickson)
Diffusion
The dictionary definition for diffusion is “the spreading of something more widely”. This is a very broad way of explaining a scientific concept, but it is also a helpful way of understanding it.
For example, have you ever been in a room where somebody puts on a fragrant lotion and at first you just see them put it on, but within a short period of time, you also can smell it? This is an example of diffusion, because the fragrance is moving from an area of higher concentration (on the person’s hands) to an area of lower concentration (throughout the room).
In science, there are a few different types of diffusion. There is simple diffusion, facilitated diffusion, and osmosis.

Simple Diffusion
Speaking on a cellular level, simple diffusion is also known as passive transport. This is the ability for a substance to pass through the cell membrane without using any energy from the cell. This does not mean that any and all substances can move through the cell membrane. Cell membranes are selectively permeable, which means that they only allow certain substances to pass through. (Khan Academy)

Facilitated diffusion
If a particle is charged for example, sodium and potassium, it will interact with the phosphate heads of the cell membrane. To make the diffusion process more efficient there are protein channels and carrier proteins that can help move these particles down its concentration gradient. These can also be gated based on the conditions that are present. (Khan Academy)


Osmosis
Osmosis is the diffusion of water to an area of higher concentration to an area of lower concentration. The goal of osmosis is to create an equal concentration throughout the water of the solute that is with it. An example of this could be making Kool-Aid. For example, when the sugar and the flavor mixture are put in the water, the solutes immediately begin to spread. To catalyze this process, we often get a spoon to stir it all together. If left alone to combine itself, it would do so over a longer period of time.

REFERENCES:
Libretexts. Laura Dickson (2023, February 13). 9: Diffusion. Chemistry LibreTexts. https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Kinetics/09%3A_Diffusion
Khan Academy. (n.d.). Simple Diffusion and Active Transport. Khan Academy. https://www.khanacademy.org/science/biology/membranes-and-transport/passive-transport/a/diffusion-and-passive-transport
Media Attributions
- Screenshot 2025-02-18 at 10.34.48 PM
- Screenshot 2025-02-18 at 11.04.24 PM
- Simple diffusion in cell membrane © LadyofHats, Villareal, Mariana Ruiz is licensed under a Public Domain license
- Facilitated Diffusion © Blausen, Bruce is licensed under a CC BY (Attribution) license
- Screenshot 2025-02-18 at 11.04.54 PM
- Screenshot 2025-02-18 at 11.10.55 PM

