Monoamines
Jackson T. Anderson; Tess Johnson; Caleb Bevan; and Jim Hutchins
Chapter under construction. This is the first draft. If you have questions, or want to help in the writing or editing process, please contact hutchins.jim@gmail.com.
Monoamines
Monoamines represent a class of neurotransmitters characterized by a single amine group (-NH₂) attached to an aromatic ring via a two-carbon side chain (-CH₂-CH₂-). The following neurotransmitters fall under the category of monoamines: histamine, dopamine (DA), norepinephrine (NE), epinephrine (EPI), serotonin (5-hydroxytryptamine; 5-HT), and melatonin (MLT). These neurotransmitters play crucial roles in various cognitive and autonomic processes.
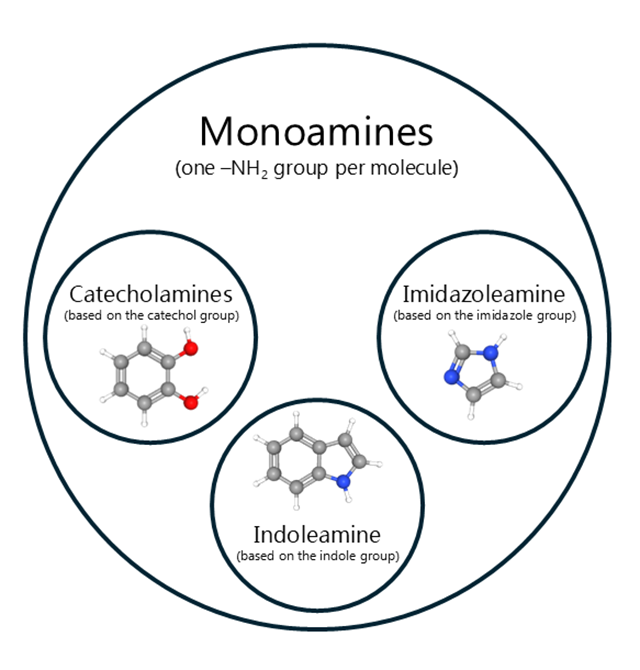
All of these compounds are synthesized from aromatic amino acids, including tyrosine (Tyr), tryptophan (Trp), and histidine, among others. The synthesis of the essential monoamines will be summarized in Figures 1 through 3.
Monoamines can be further categorized based on their precursor amino acids into distinct groups such as imidazole amines, catecholamines, and indoleamines (see Table 1).
Table 1. Essential Aromatic Amino Acids and Their Products
| Monoamine Category | Aromatic Amino Acid | Derived Monoamine(s) | Function(s) |
|---|---|---|---|
| Catecholamines | Tyrosine (Tyr) | Dopamine (DA) | Reward, Motivation, Stress Response, Autonomic Mechanisms |
| Imidazoleamine | Histidine | Histamine | Wakefulness, Immune response, Gastric Acid Secretion |
| Indoleamines | Tryptophan (Trp) | Serotonin (5-HT)
Melatonin (MLT) |
Mood regulation, sleep, appetite, cognition |
Note. Please refer to the linked chapters in the table for a more comprehensive discussion of each.
Catecholamines
Catecholamines are a subclass of monoamines characterized by the presence of a catechol group, which consists of a benzene ring with two hydroxyl groups. When it comes to essential catecholamines, three key players stand out: DA, NE, and EPI.
Dopamine
All of the compounds above are synthesized from the amino acid tyrosine, 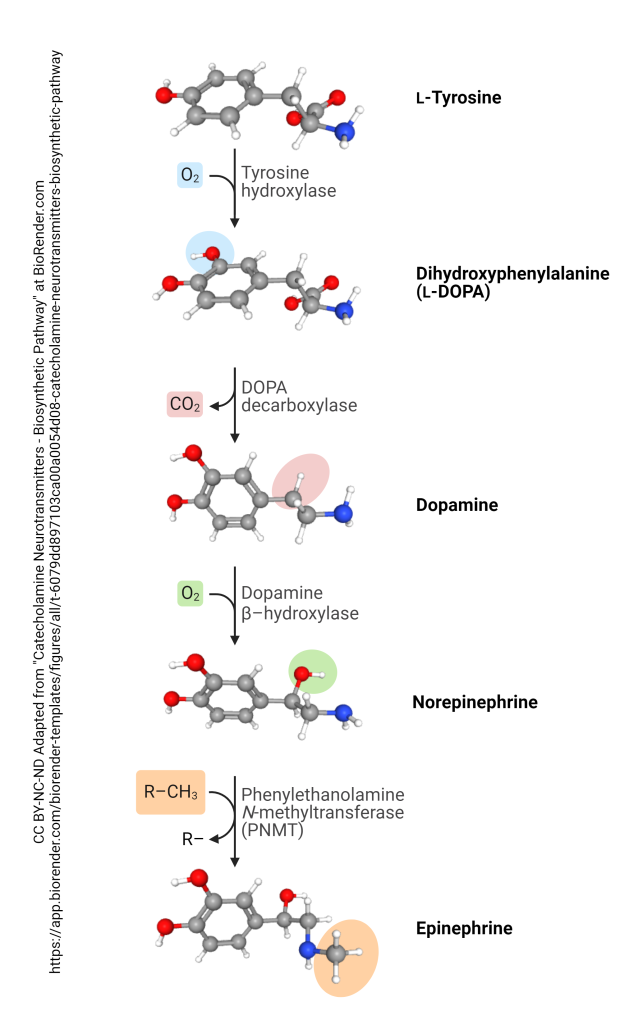
which is derived from the aromatic amino acid phenylalanine. The enzyme phenylalanine hydroxylase catalyzes the conversion of phenylalanine to tyrosine. Once phenylalanine is transformed into tyrosine, it undergoes a critical enzymatic reaction.
Tyrosine Hydroxylase (TH), a member of the Aromatic Amino Acid Hydroxylases (AAAH) family, catalyzes the hydroxylation of tyrosine, converting it into the dopamine precursor known as levorotatory DOPA (L-3,4-dihydroxyphenylalanine; L-DOPA). This reaction is considered the rate-limiting step in dopamine synthesis, as the activity of TH directly influences dopamine production in dopaminergic neurons. Following this step, L-DOPA is decarboxylated by Aromatic Amino Acid Decarboxylase (AAAD, also known as DOPA decarboxylase) to yield DA.
TH, along with phenylalanine hydroxylase and tryptophan hydroxylase, is part of the AAAH family and requires cofactors such as tetrahydrobiopterin (BH4) and molecular oxygen to fulfill its catalytic function. The hydroxylation of tyrosine by TH is meticulously regulated through mechanisms such as feedback inhibition by dopamine itself and phosphorylation by various kinases, ensuring the controlled production of dopamine.
Norepinephrine and Epinephrine
Norepinephrine and its closely related counterpart, epinephrine, are just one or two steps ahead in the potential outcomes of catecholamine synthesis. Within noradrenergic and adrenergic neurons, dopamine (DA) is converted into norepinephrine (NE) through the action of dopamine-β-hydroxylase in the adrenal medulla of the kidneys. Norepinephrine is subsequently methylated by phenylethanolamine-N-methyltransferase (PNMT) to produce epinephrine, which is then released directly into the bloodstream.
Beyond their roles as neurotransmitters, catecholamines also function as hormones that regulate autonomic physiological processes, including the stress response, arousal, and cardiovascular function. These catecholamines exert their effects by binding to a subfamily of G-protein-coupled receptors (GPCRs), specifically the adrenergic α- and β-receptors. Stimulation of α-adrenergic receptors leads to vasoconstriction in several muscles, including the radial smooth muscle of the iris, arteries, arterioles, veins, urinary bladder, and the sphincter of the gastrointestinal (GI) tract. Activation of β1-adrenergic receptors enhances myocardial contractility, increases heart rate, increases automaticity, and facilitates atrioventricular (AV) conduction. Meanwhile, β-2 adrenergic receptor stimulation induces dilation of bronchiolar and vascular smooth muscles. The α-1 and α-2 adrenergic receptors, to which noradrenaline binds, are responsible for causing vasoconstriction. By antagonizing these α-1 and α-2 receptors, there is an increase in blood pressure (BP) and an increase in systemic vascular resistance. [1]
Imidazoleamines
Histamine
Neurotransmitters Resembling Monoamines
Acetylcholine
Adenosine
Peptides
Media Attributions
- Monoamine venn diagram © PubChem adapted by Jim Hutchins is licensed under a CC BY-SA (Attribution ShareAlike) license
- For a more comprehensive discussion, please refer to the following chapter on Norepinephrine and Epinephrine. ↵

