Neurotransmitters: Noradrenaline
Abbigail Higgins
This is a first draft which is in the process of being edited. If you have questions, or want to help in the writing or editing process, please contact jimhutchins@weber.edu or the author: abbigailhiggins@mail.weber.edu
Noradrenaline Production and Packaging
Noradrenaline, also known as Norepinephrine (NE), is a critical neurotransmitter for many brain functions. Before we can get too far into its neurological interactions, it is important to understand how we get noradrenaline in the body. Noradrenaline is a part of a chain of reactions (pictured bellow, and in Monoamines and Catecholamines) that takes L-Tyrosine to Epinephrine. Because of this chain, the quantities of Norepinephrine are impacted by the levels of L-Tyrosine, L-DOPA, Dopamine, and Epinephrine. Changes in the molecule or enzyme concentrations will often slightly alter the rate of reaction (if there is not much of something, it will be much harder to get the reaction to occur and will slow things down, and vise versa). Particularly, the conversion of L-Tyrosine to L-DOPA by Tyrosine hydroxylase (TH) is the most impactful on the rate of reaction. This is because it is the slowest step (the rate-limiting step), so no mater how fast the other reactions are able to go they will always be waiting on the first step (the most difficult hill to get over). Because of Tyrosine hydroxylase’s function in the rate limiting step, it is used by the cell in the regulation of chatecolamines. This happens at just about every point in its existence, from translation and transcription to its activity in the cell, including end product inhibition with NE and DA as well as through Ca++ and cAMP.
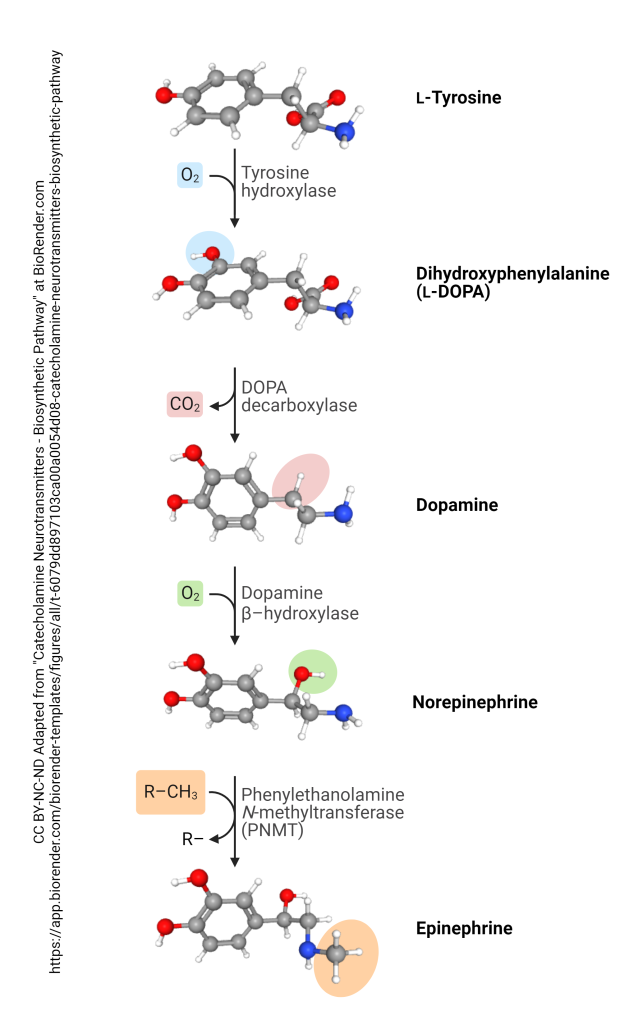
Synthesis from Dopamine to Norepinephrine occurs with the use of Dopamine-β-hydroxylase (DBH). This enzyme adds a hydroxyl (-OH) group to the carbon beta of Dopamine’s functional amine (hence the name, Dopamine-β-hydroxylase). This enzyme is located in noradrenergic/adrenergic neurons of the brain, as well as the adrenal medulla and sympathetic neurons. As such, norepinephrine is primarily produced in the adrenal medulla and brain stem, as well as various locations throughout the body.
Once Norepinephrine is created and tagged to be sent out, it is then packaged into membrane bound vesicles. This can be in chromaffin granuals (within adrenal medula cells), which allow long term storage (greater than a day), or in neuronal vesicles for shorter storage (with a half time between 3-43 minutes). From there, Vesicular Monoamine Transporters (VMATs) are able to transfer the Norepinephrine to its target location. VMAT comes in two forms, VMAT1 which is primarily in neuroendocrine cells and VMAT2 which is expressed most in the CNS. Both are able to move Norepinephrine, with some small differences in affinity.
Norepinephrine Receptors

Norepinephrine acts on metabotropic receptors (see Neurotransmitter Receptors and Comparing Ionotropic and Metabotropic Receptors). There are tree main adrenergic receptor types, all with subtypes, that play important roles in neuronal function. These types all play a role in the hypothalamus within ascending arousal pathways, and also have many more differing functions.
Alpha 1 receptors
Alpha 1 (α1) receptors primarily target Gq/G11 . This activates phospholipase C, which increases Ca+ and protein kinase C activation, and also activates phospholipase D and mitogen-activated protein (MAP) kinase. These effects lead to wakefulness during stimulation, and α1 receptors also play a role in the amygdala and hypocampus. High activation of α1 receptors in the prefrontal cortex may however impare its functions. Alpha 1 activation can also lead to smooth muscle contraction, with various effect through the body. There are three subtypes of α1, being α1A, α1B, and α1D. All three function the same, however have some small differences in structure and have differing locations through the body. Within the brain specifically, type α1A is found in the cerebellum and cerebral cortex and α1D is found in the cerebral cortex.
Alpha 2 receptors
Alpha 2 (α2) activation primarily targets Gi/Go which inhibits adenylyl cyclase (decreasing cAMP) and stimulates phospholipase A2 activity, as well as leading to activation of K+ channels and inhibition of Ca+ channels. This can lead to an enhancement of prefrontal cortex function, as opposed to α1 receptors. Alpha 2 activation can also lead to smooth muscle contraction, with some slightly different effects than α1 due to location. Alpha 2 receptors are present both presynaptic and postsynaptic, unlike the other receptors which are only present postsynaptic. There are also 3 subytpes of α2 receptors, being α2A, α2B, and α2C. Again, these three have some structural and location differences, but all still have the same functions. Within the brain specifically, α2A are found in the locus ceruleus and hippocampus, α2B in the thalamus, and α2C are found in the hippocampus and olfactory bulb.
Beta 1, 2, and 3 receptors
Beta (β) receptors are a bit less varied from one another than alpha receptors, and all subtypes target Gs to activate adenylyl cyclase activity (increasing cAMP), which then activates protein kinase A. This activation plays a role in heart contraction (β1), and β2 activation plays an important role in bronchodilation. Beta receptors also have various effects in relaxing smooth muscle around the body (with each receptor type varying based on location). In the brain specifically, β1 is found in the cerebral cortex and hypothalamus, β2 is found in the cerebellum, hippocampus, cerebral cortex, and olfactory bulb, and β3 is found in the hypothalamus and hypocampus (locations still being researched).
Norepinephrine Re-uptake and Removal
[Add diagram]
Once Norepinephrine is chilling out in the synapse and no longer needed, it must be taken back into the cell. Norepinephrine reuptake is completed primarily with an enzyme called Norepinephrine Transporter (NET). Many medications act on NET, including stimulant and non-stimulant medications used to treat ADHD and narcalepsy as well as Bupropion for depression (these all being examples of inhibitors, though there are a few activators used for different purposes). Amphetamine medications are known to both inhibit reuptake and reverses transporters to further increase NE in the synapse. Since ADHD is heavily associated with lower levels of Norepinephrine, increasing its concentration in neurons is often used as treatment.
After reuptake, with NE is back in the cell, it is packaged back into synaptic vesicles. Then it can either be reused or sent to be broken down. Monoamine oxidase (MAO) is the main enzyme for breakdown of many different neurotransmitters. MAO comes in two isoforms, with MAO-A having greater affinity for NE than MAO-B. After NE breakdown is begun by MAO, it then gets broken down further by catechol o-methyltransferase (COMT). After a couple more breakdown steps it finally becomes vanillymandelic acid to be excreted through the urine.

