The Cyclic Guanosine Monophosphate (cGMP) Second Messenger System
Brennan Brown
This is a work in progress. If you have questions, or want to help in the writing or editing process, please contact the author: jimhutchins@weber.edu
As a central signaling cascade in the nervous system, the cyclic guanosine monophosphate (cGMP) second messenger system regulates a wide scope of cellular and molecular functions that are essential for neuronal function.
In its basic form this system is based on the synthesis, action and degradation of cGMP, a cyclic nucleotide produced from guanosine triphosphate (GTP). The production of cGMP is mainly through the action of guanylate cyclase’s, enzymes that are divided into two major classes: soluble guanylate cyclase (sGC) and particulate guanylate cyclase (pGC). sGC, a cytosolic enzyme, is activated by nitric oxide (NO), a gas neurotransmitter that can diffuse. When NO combines with sGC, the enzyme changes its configuration and boosts cGMP production. This NO-sGC-cGMP pathway is also involved in synaptic plasticity and affects such processes as LTP and LTD, which are learning and memory related. On the other hand, pGC is a membrane bound receptor which is stimulated by natriuretic peptides and is involved in water and electrolyte management in the brain and other neuromodulator actions.
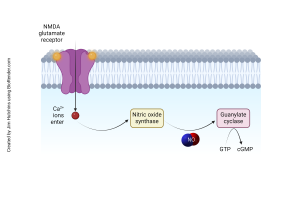 Once formed, cGMP exerts its effects through several key molecular targets that are available in neurons. The most important of these is protein kinase G (PKG), a serine/threonine kinase that is the main effector of the cGMP signaling. PKG phosphorylates a multitude of proteins that regulate their function. Neurons contain PKG that plays an important role in regulating ion channels such as potassium and calcium channels thereby controlling the excitability of the cell.
Once formed, cGMP exerts its effects through several key molecular targets that are available in neurons. The most important of these is protein kinase G (PKG), a serine/threonine kinase that is the main effector of the cGMP signaling. PKG phosphorylates a multitude of proteins that regulate their function. Neurons contain PKG that plays an important role in regulating ion channels such as potassium and calcium channels thereby controlling the excitability of the cell.
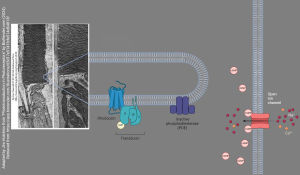
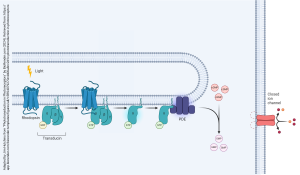
 It also regulates transmitter release by altering the processing of synaptic vesicles and participates in cytoskeletal regulation of axonal growth and dendritic spine formation. Also, PKG can affect gene expression by cAMP-dependent phosphorylation of transcription factors and, therefore, affect the long-term modification of neuronal function. Moreover, cGMP can directly activate cGMP-gated ion channels; these are especially important in photoreceptor cells of the retina where they are responsible for the light-induced hyperpolarization response. In other neuronal situations, these channels can admit Ca2+, and lead to membrane depolarization.
It also regulates transmitter release by altering the processing of synaptic vesicles and participates in cytoskeletal regulation of axonal growth and dendritic spine formation. Also, PKG can affect gene expression by cAMP-dependent phosphorylation of transcription factors and, therefore, affect the long-term modification of neuronal function. Moreover, cGMP can directly activate cGMP-gated ion channels; these are especially important in photoreceptor cells of the retina where they are responsible for the light-induced hyperpolarization response. In other neuronal situations, these channels can admit Ca2+, and lead to membrane depolarization.
The regulation of the time and specific regions of cGMP signaling is carried out by phosphodiesterase (PDEs) – enzymes that break down cGMP, thus canceling its action. There are various PDE isoforms, which have different substrate selectivity and express in different tissues, thus allowing for the spatial control of cGMP levels. In the nervous system, PDEs are involved in the control of the extent and the frequency of the cGMP signals.
The second messenger system of cGMP is involved in synaptic plasticity and neurotransmission. In the LTP and LTD, the NO-sGC-cGMP pathway controls the strength of the synapse through the control of the release of neurotransmitter form the presynaptic terminal and the reception of the neurotransmitter in the postsynaptic terminal. Among them, PKG is involved in the removal or insertion of AMPA receptors at the postsynaptic membrane to control the strength of the synapse. Furthermore, cGMP can regulate the probability of neurotransmitter release by affecting calcium influx into the presynaptic terminal. PKG can regulate vesicle docking and fusion proteins, and therefore the degree of synaptic transmission. However, synaptic plasticity cGMP is also involved in axonal guidance and dendritic branching in neuronal development. It can also regulate the expression of cytoskeletal proteins thereby determining the shape and number of connections of neurons.
 The cGMP signaling pathway is involved in various neurological disorders and their pathogenesis. In stroke, the NO-sGC-cGMP pathway is protective, dilates blood vessels, and reduces neuronal injury. However, there is also the possibility of NO overproduction that can lead to excitotoxicity, which again stresses the importance of the correct cGMP signaling levels. In Alzheimer’s disease, the cGMP signaling is altered and some studies suggest that increasing cGMP levels may be beneficial. The NO/cGMP pathway is also important in the function of basal ganglia and is, therefore, involved in Parkinson’s disease. Mutations in cGMP-related genes can cause retinal degeneration, including retinitis pigmentosa, which highlights the importance of cGMP in vision.
The cGMP signaling pathway is involved in various neurological disorders and their pathogenesis. In stroke, the NO-sGC-cGMP pathway is protective, dilates blood vessels, and reduces neuronal injury. However, there is also the possibility of NO overproduction that can lead to excitotoxicity, which again stresses the importance of the correct cGMP signaling levels. In Alzheimer’s disease, the cGMP signaling is altered and some studies suggest that increasing cGMP levels may be beneficial. The NO/cGMP pathway is also important in the function of basal ganglia and is, therefore, involved in Parkinson’s disease. Mutations in cGMP-related genes can cause retinal degeneration, including retinitis pigmentosa, which highlights the importance of cGMP in vision.
 To gain a better understanding of the cGMP second messenger system, a number of sophisticated tools and techniques are used by researchers. Using fluorescent sensors, cGMP imaging enables the visualization of cGMP levels in living cells, and thereby the understanding of the time-course and the spatial patterns of cGMP signaling. Gene knock-out and knock-in techniques are used to understand the role of different components of the cGMP signaling pathway in neuronal function. Secondary compounds, including PDE inhibitors and sGC agonists, are useful in the modulation of cGMP signaling to help understand its effects. The effect of cGMP on neuronal excitability and synaptic transmission is studied with electrophysiological techniques like patch-clamp recording. These diverse approaches make it possible for researchers to determine the multifarious roles of the cGMP second messenger system in neuronal function and dysfunction and, thus, to design new strategies for the treatment of neurological disorders.
To gain a better understanding of the cGMP second messenger system, a number of sophisticated tools and techniques are used by researchers. Using fluorescent sensors, cGMP imaging enables the visualization of cGMP levels in living cells, and thereby the understanding of the time-course and the spatial patterns of cGMP signaling. Gene knock-out and knock-in techniques are used to understand the role of different components of the cGMP signaling pathway in neuronal function. Secondary compounds, including PDE inhibitors and sGC agonists, are useful in the modulation of cGMP signaling to help understand its effects. The effect of cGMP on neuronal excitability and synaptic transmission is studied with electrophysiological techniques like patch-clamp recording. These diverse approaches make it possible for researchers to determine the multifarious roles of the cGMP second messenger system in neuronal function and dysfunction and, thus, to design new strategies for the treatment of neurological disorders.
ENTER VISION SECTION HERE

