Overview of Membrane Channels, Transporters, and Pumps
Jaron Williams; Avalon Marker; and Jim Hutchins
Chapter under construction. This is the first draft. If you have questions, or want to help in the writing or editing process, please contact hutchins.jim@gmail.com.
In this chapter, we will give a brief overview of the many channels, transporters, and pumps found in the cell membrane. As these will be discussed in further detail in later chapters, this chapter is not meant to provide explicit details nor is it meant to be an exhaustive list. This chapter will provide a brief introduction and description of each membrane protein type and will help you become familiar with the functions of them.
Membrane Permeability
The cell membrane of a neuron is selectively permeable, meaning that it will let some molecules in freely while others will require a more difficult entry. Molecules such as gases, lipid-soluble molecules such as steroids, and water are all free to enter and exit as they choose using diffusion. All other molecules, especially ions (those with an electrical charge), require some form of assistance to enter or exit a neuron. Often these molecules are moved against their concentration gradient and thus require the use of active transport. To do this, the cell membrane contains a number of membrane proteins in the form of pumps, transporters, and channels to allow molecules into the cell while sending others out, according to the needs of the neuron. As we will see these proteins are important in maintaining proper concentration gradients of various molecules integral to neuronal function.
Channels
The main category of membrane proteins used to help move molecules across the cell membrane are known as channels. Channels are membrane proteins that span the length of the cell from extra to intracellular , otherwise known as integral proteins. In general, channels are picky, they prefer to only transport one specific molecule across the membrane. Because of this, there are many different types of channels across the cell membrane. However, channels move molecules at a fairly high rate There are two major categories of channels, gated and ungated. In this section, we will focus on 4 major types of channels, leakage, voltage-gated, ligand-gated, and signal gated channels.
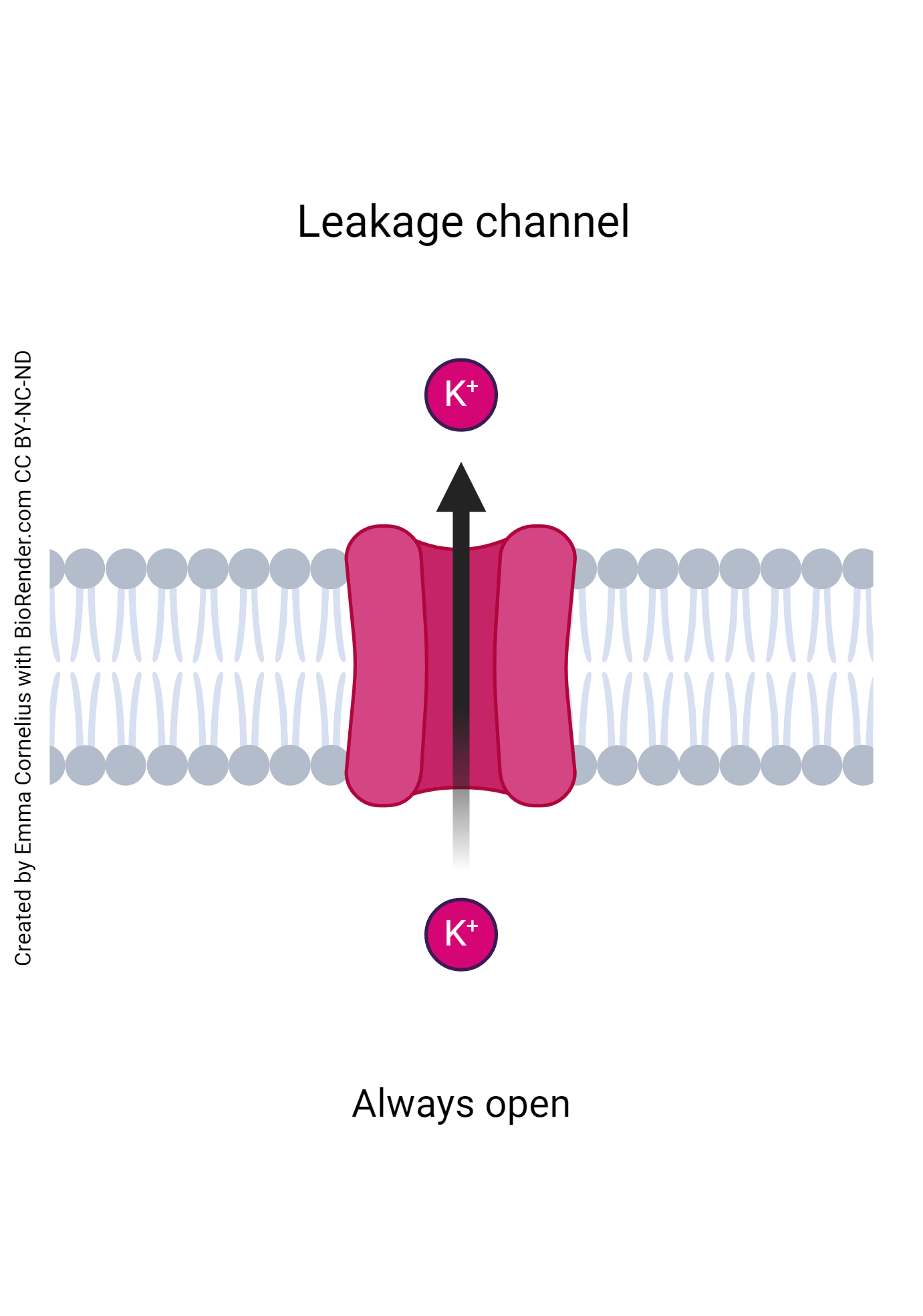
- Leakage Channels: as the name implies, leakage channels, or sometimes referred to as ungated channels, are constantly open for specific ions. The most common leakage channels are potassium and sodium leakage channels. This is due to these ions being heavily involved in membrane action potential. With these molecules moving so frequently, it saves the cell time and energy to leave these channels constantly open.
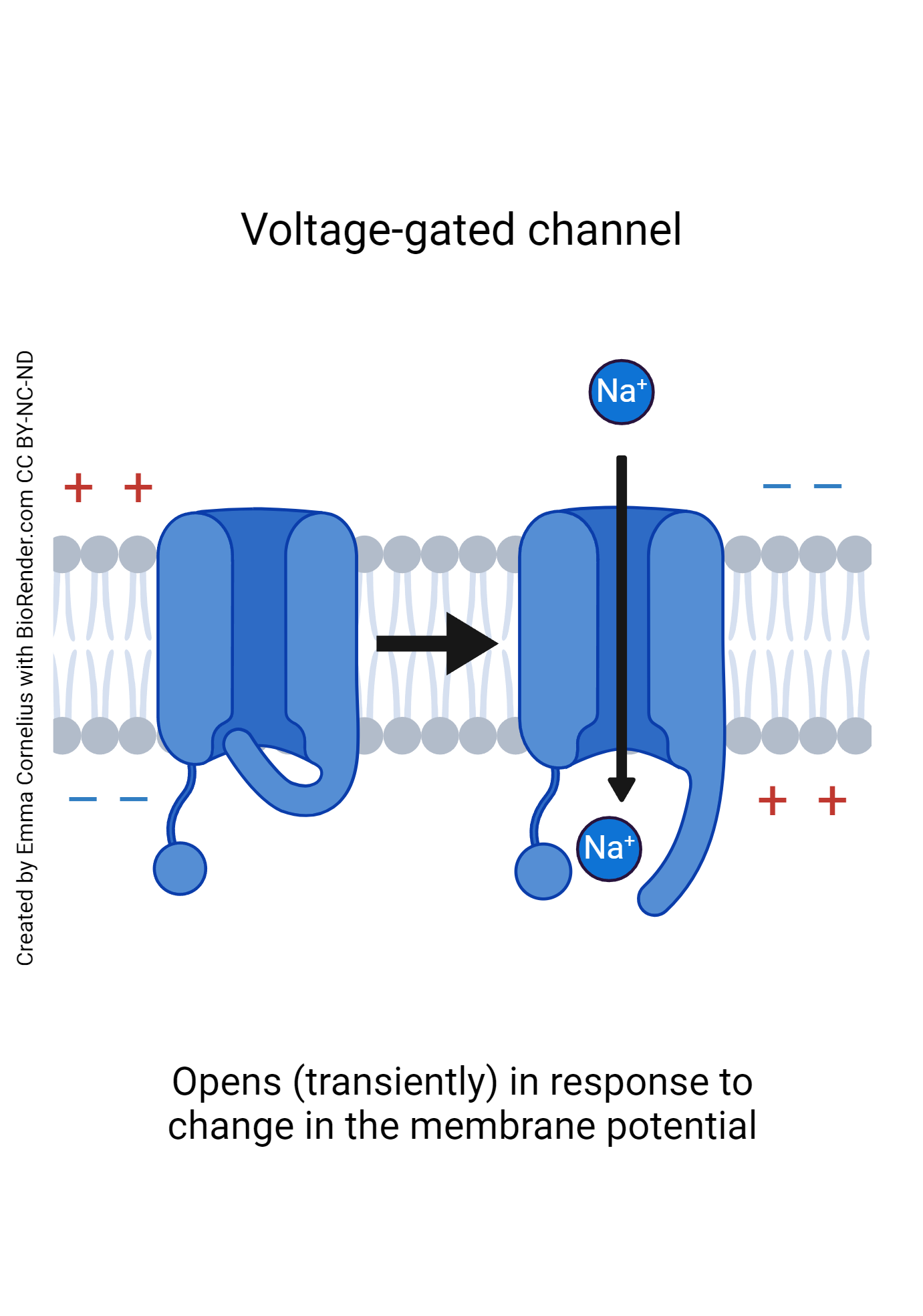
- Voltage gated channels : While leakage channels are essentially always open, voltage gated channels are a little more selective. Voltage-Gated channels open in response to a change in membrane voltage. When the membrane changes voltage, the channel protein will respond by altering its shape to either open, resting state closed, or inactivated state closed configuration depending on the needs of the neuron.
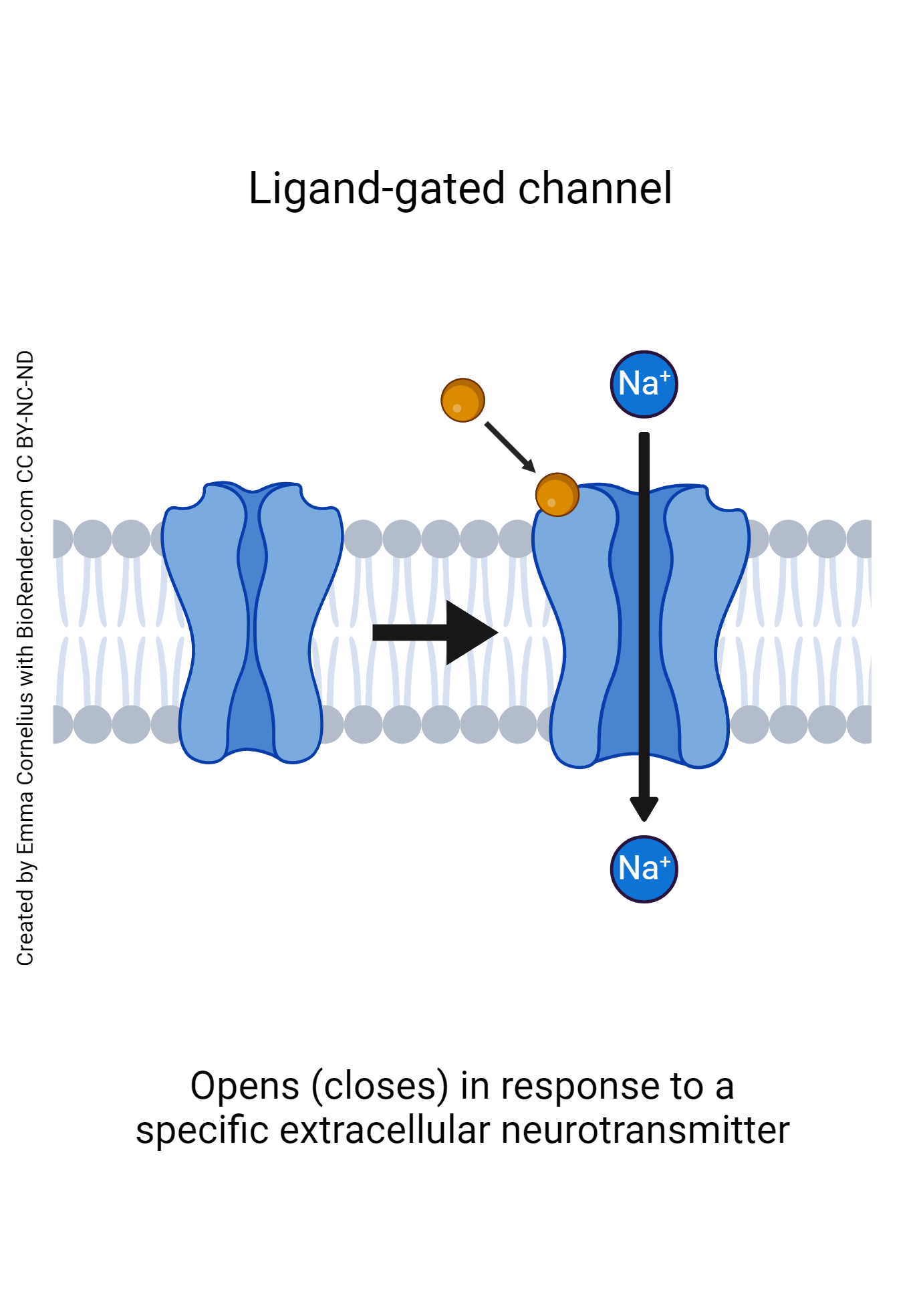
- Ligand-gated channels: a ligand is a term used to describe any molecule that binds to a receptor. When referring to neurons, these ligands are almost always a neurotransmitter. Ligand-gated channels have special receptors that bind to specific neurotransmitters. Like voltage-gated channels, ligand-gated channels change shape when activated, the difference being that ligand-gated channels are activated by a binding molecule as opposed to a change in voltage.
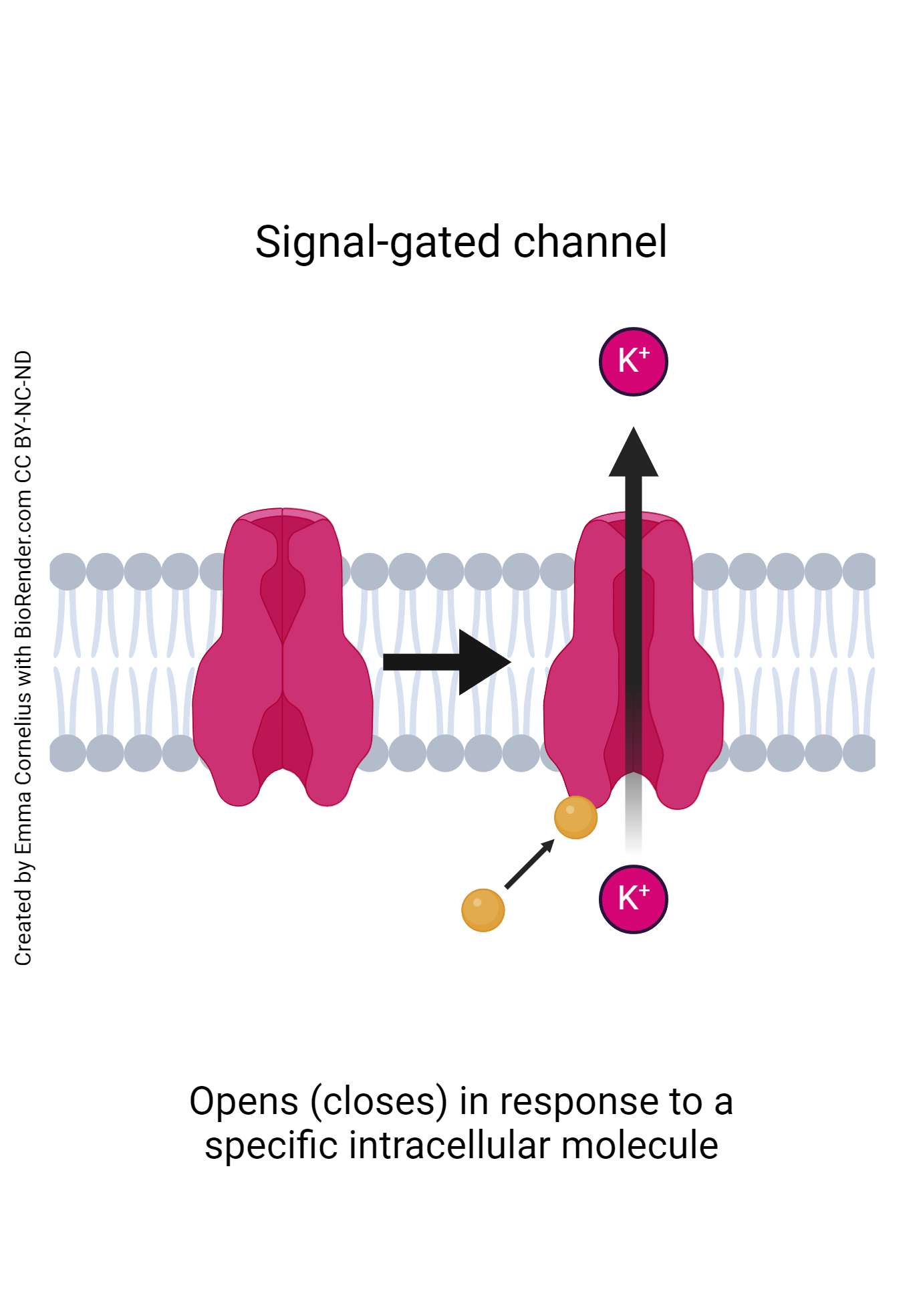
- Signal-gated channels: Up until now, each form of channel has been activated by extracellular molecules or changes in membrane voltage. Signal gated channels, however, differ in that they are activated by the presence of an intracellular molecule. The process is relatively similar to that of ligand-gated channels but the activating ligand is a molecule already present within the cell. As with the other forms of channels, signal-gated channels only respond to one specific molecule. As such the cell membrane is full of many different types of signal-gated channels, each designed for a specific ligand.
Transporters
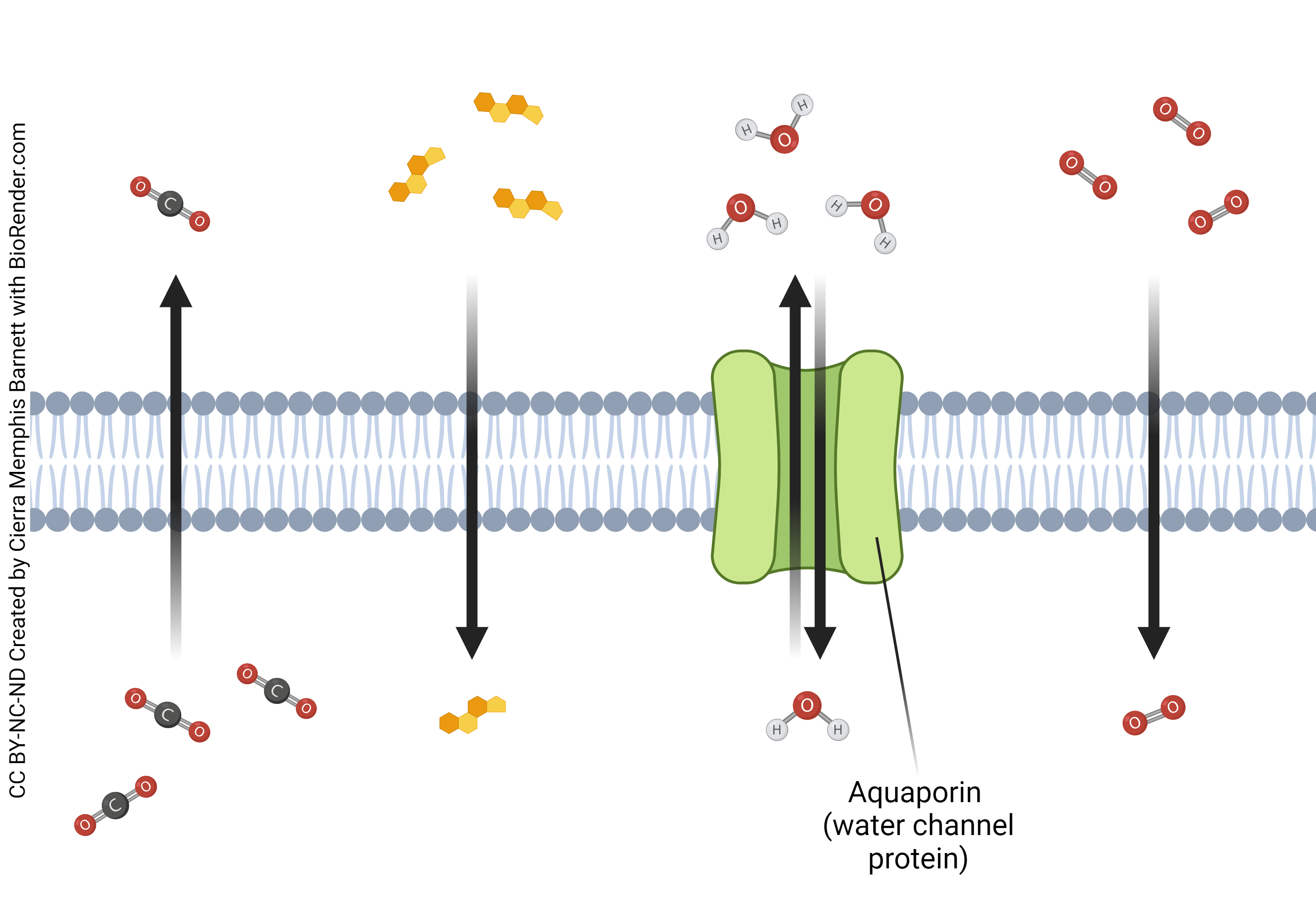
As the name implies, transporters transport ions from inside to outside of the cell or vice versa. In order to accomplish this, transporters may use passive or active transport. They differ from channels in that they can move molecules against their concentration gradient as opposed to passively with their gradient, which requires the use of ATP. Additionally, transporters move molecules at a much slower rate than channels do. While not all transporters will use active transport many do, which is why transporters are critical in maintaining proper concentration gradients of molecules
- Categories: There are three main categories of transporters, symporters (cotransporters) ,antiporters or (exchangers), and uniporters. Symporters move two molecules in the same physical direction, either inside or outside of the cell. Symporters can move different molecules or the same type of molecule. Antiporters function by sending one molecule in one direction while sending a different molecule in the opposite direction. Like symporters, these can be the same or different types of molecules. Uniporters move a single molecule in one physical direction, either inside or outside of the cell. The image below will help to give a visual representation of how these different transporters operate.
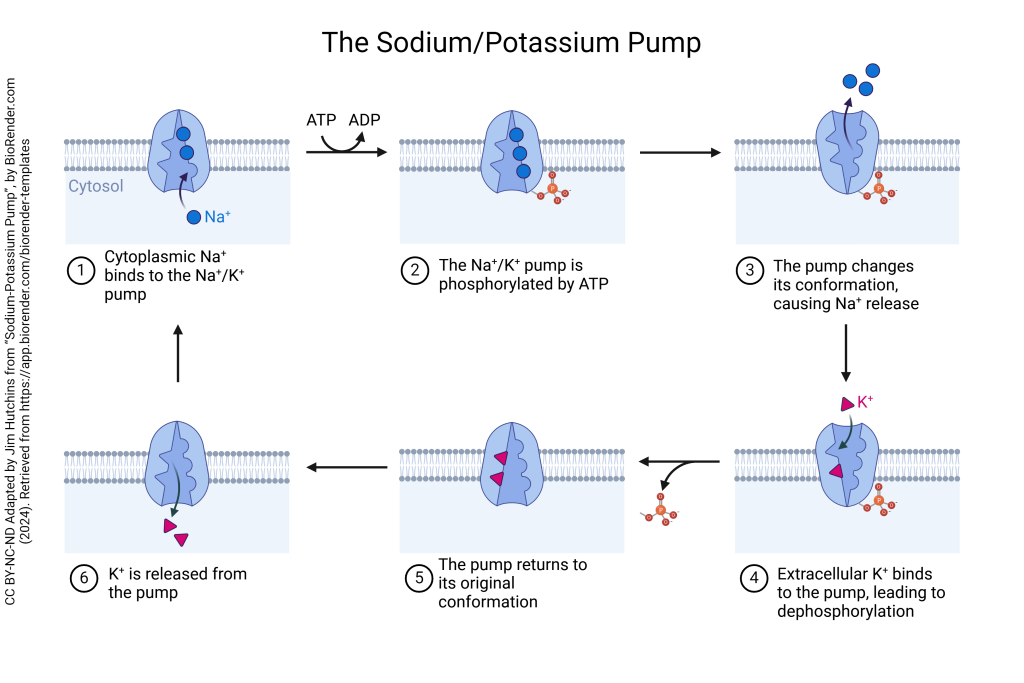
Pumps
These membrane proteins differ from transporters in that they are always using active transport to move molecules, but the underlying function is the same for both. While transporters make use of both passive and active transport mechanisms, pumps are strictly operated using active transport. In essence, all pumps are transporters but not all transporters are pumps. Similarly to transporters, pumps can move the same or different molecules in either the same physical direction or in opposing directions. Regardless of what molecules are being moved they are always done so against their concentration gradient, which requires the use of ATP. The most common and frequently used pump is known as the sodium-potassium pump, which will be discussed in detail in the chapter on pumps.