Platelets and Hemostasis
Objective 5
Describe the production and function of platelets. Define hemostasis and describe the three mechanisms that contribute to hemostasis. Describe the process of coagulation: stimuli, pathways, and products. Explain the purpose and basic functioning of the fibrinolytic system.
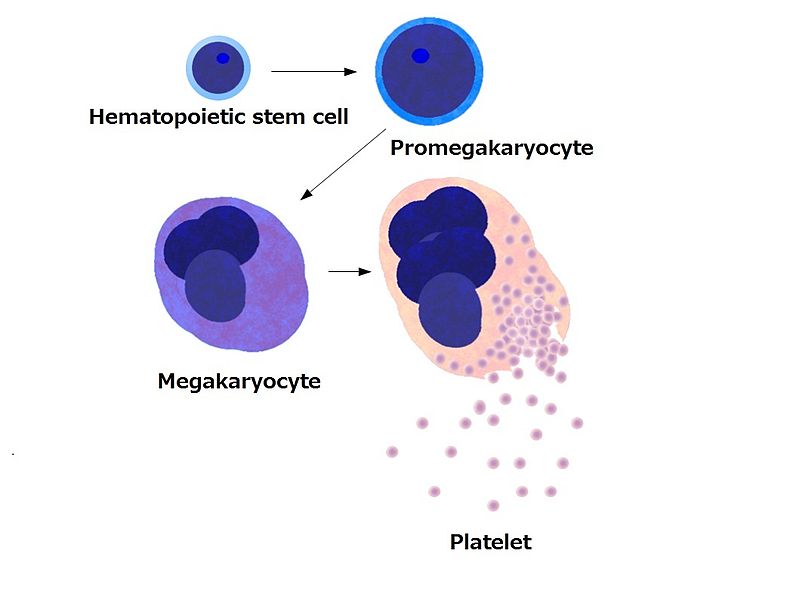
Platelets, also called thrombocytes, are cytoplasmic fragments of large cells in the bone marrow called megakaryocytes. These cells are 10-15 times larger than a red cell, and due to their size, they don’t escape the bone marrow and show up in the blood stream. An individual megakaryocyte can release 2000-5000 platelets. The normal range for platelets in the blood is 150–400 × 103/ µL.
Platelets have a very short lifespan, approximately 5–9 days. Platelets help limit blood loss by forming a platelet plug and releasing chemicals to encourage vasoconstriction and activate the clotting process.
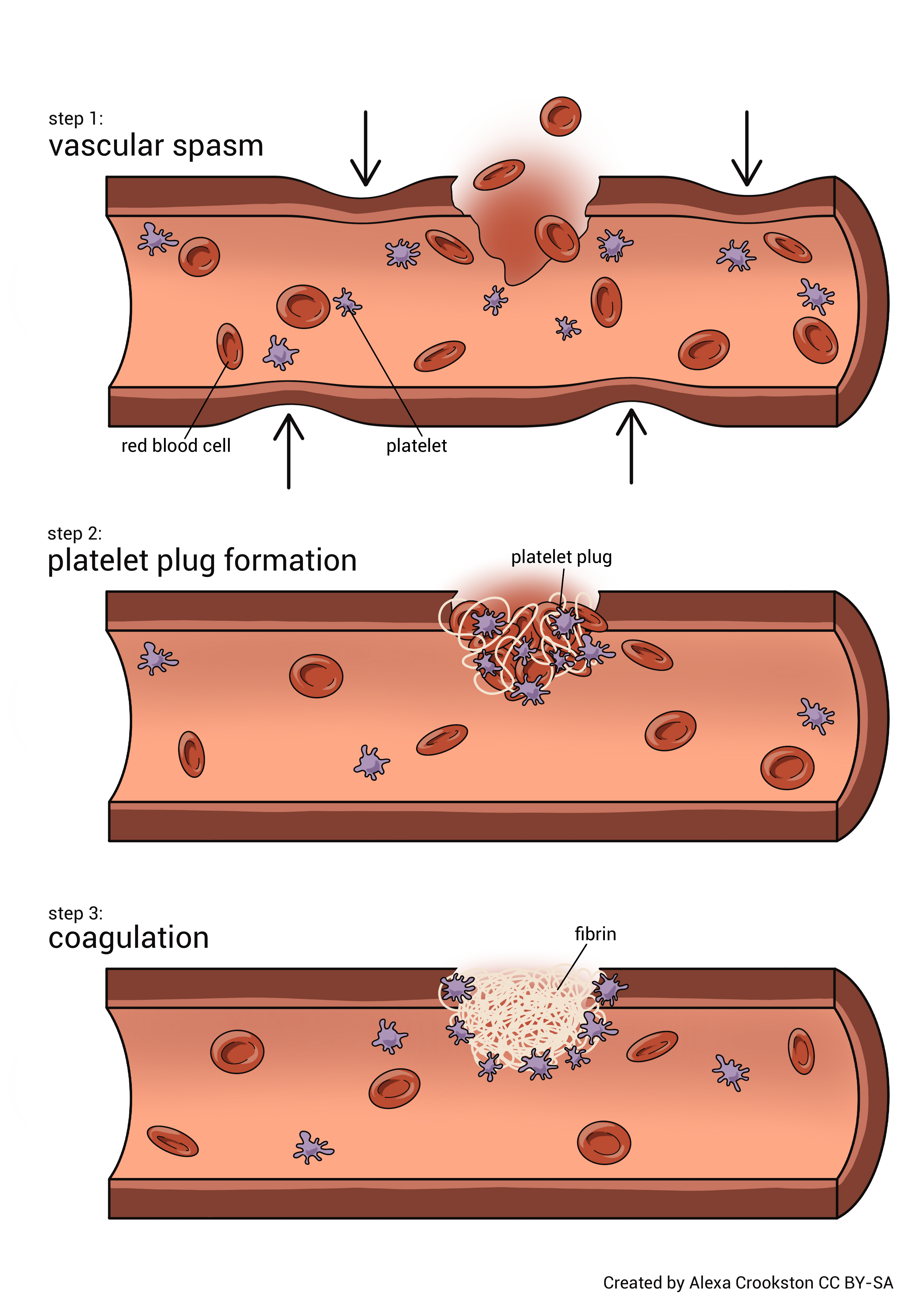
The term hemostasis, not to be confused with homeostasis, has a Greek origin, meaning blood stagnation, or blood stopping. It is a very complex process. When activated, the process causes bleeding to cease. There are three mechanisms involved: vascular spasm, platelet plug formation, and coagulation.
Vascular spasm is the constriction of damaged blood vessels. This limits the amount of blood lost. The vascular constriction is due to chemicals released from platelets, damage to the smooth muscle of the vessels, and pain receptor reflexes.
Platelets are very active in the hemostatic process. Think of them as little bags of procoagulant chemicals. Platelets form a plug through a three-step process. First, the platelets adhere to the wall of the blood vessel. Second, the platelets release their chemical contents. This encourages further vasoconstriction and recruitment of other platelets. Lastly, due to chemical release, the activated and newly-recruited platelets become sticky. The clumping of platelets is called platelet aggregation. With the activation of enough platelets, a loose platelet plug is formed.
Everyone witnesses this event sometime in their life, some more than others. When someone cuts themselves shaving, it is common practice to place a piece of toilet paper over the cut. This keeps blood off their clothing and it makes an attempt at stopping the bleeding. Because the individual is usually in a hurry, (that is why they cut themselves in the first place) they remove the toilet paper prior to going out into public. They are disappointed to find out that they begin bleeding again. What happened to the platelets? They are on the toilet paper, so there isn’t an intact platelet plug. Now, that is embarrassing.
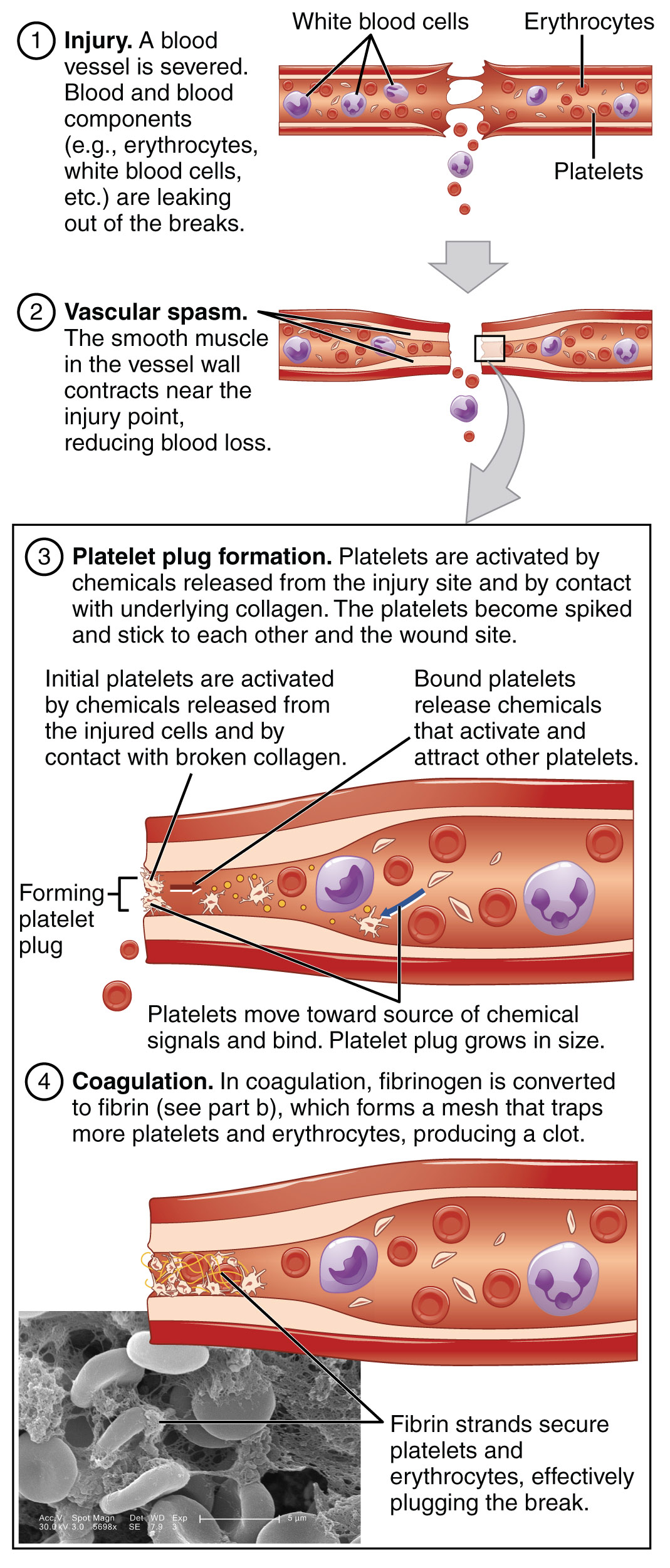
The last mechanism in hemostasis is coagulation. An easy way to think of coagulation is taking that which is liquid (plasma) and make it a solid (clot) or at least a semi-solid.
Coagulation is a complex series of enzymatic reactions that occur in a stepwise or cascading fashion. Think of the steps as dominos standing next to each other, ready to fall. When injury occurs, it tips over the first domino. Just like tipping over the dominos, all of the dominos have to be in place to get to the end. If one is removed, the process stops.
The dominos in this game are called clotting or coagulation factors. Once one is activated, the new active form stimulates the next factor, which activates the next, and so on. Most of these factors are synthesized by the liver and circulate in an inactive form. They are enumerated with Roman numerals (factors I, III, IV, X, etc.), in addition to having specific names (fibrinogen, tissue thromboplastin, calcium prothrombinase etc.)
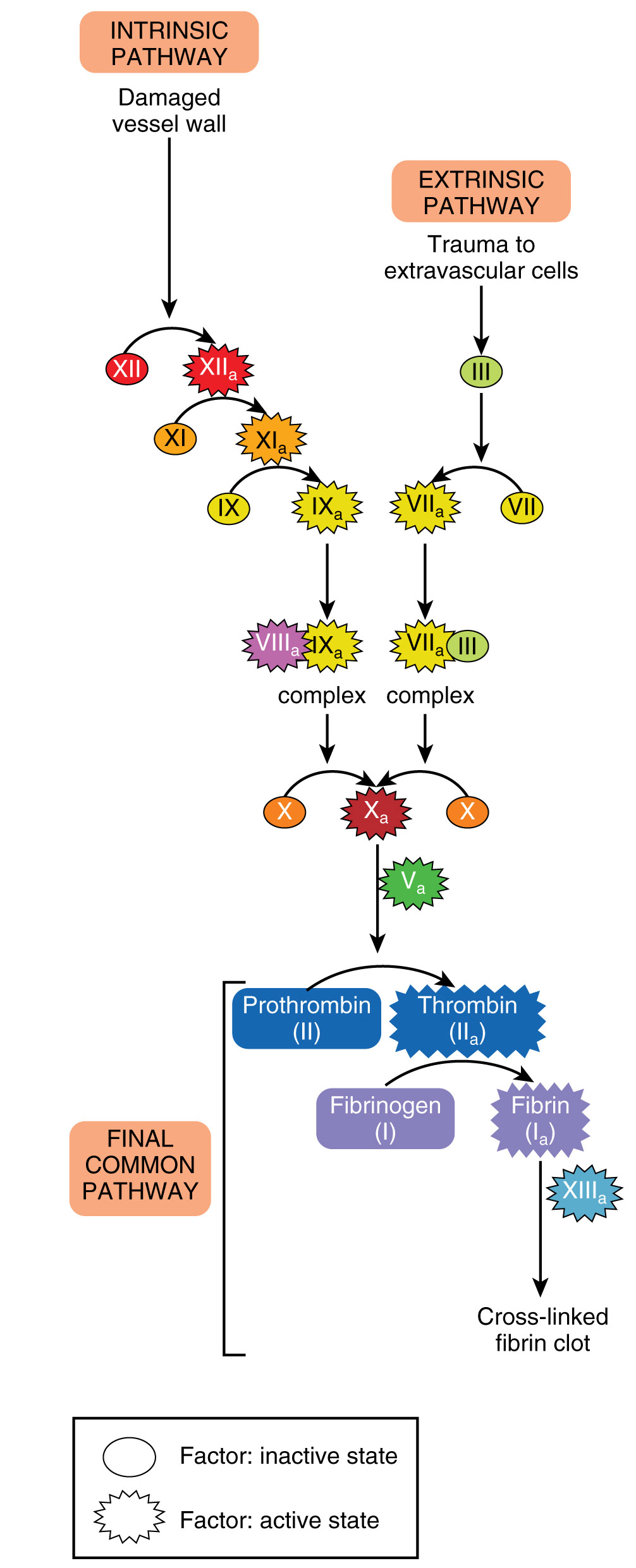
There are two separate pathways to activate coagulation, and they merge to form a common pathway. A person may ask why there is a need for two different activation pathways. The presence of two pathways attempts to guarantee that bleeding stops. Each of the two pathways is activated in a slightly different fashion, and the name gives a hint to their activator.
The extrinsic pathway is activated by damage outside of the vessel, specifically to the tissue. A tissue protein called tissue factor (TF) or tissue thromboplastin is released from the damaged tissue into the blood vessels. The extrinsic pathway has fewer steps and occurs more rapidly than the intrinsic pathway. This functions like feeling a draft coming into your house on a cold day. You can follow where the air is coming in from to find the window that is open or broken. Similarly, the presence of tissue thromboplastin indicates to cells and proteins in the vessel that there is a hole that needs to be closed.
The intrinsic pathway is activated by substances within or associated with the vessel. Exposed vascular collagen, damaged endothelium, or activated platelets are all potent intrinsic pathway activators.
Regardless of the pathway, the goal is to merge at a common pathway by activating an enzyme called prothrombinase (Factor X). Prothrombinase acts on prothrombin (factor II) to create thrombin. The newly formed thrombin will activate fibrinogen (factor I) to create fibrin. Fibrin proteins are the threads that form the clot and strengthen the platelet plug. In addition to the factors mentioned, a number of cofactors are required to allow clotting to take place. Vitamin K and calcium are among the most important. The final product, called a thrombus or more simply, a clot, is a clump of fibrin strands woven around platelets and red blood cells. Anticoagulants are named such because they can block these steps or remove a vital factor or element needed to clot.
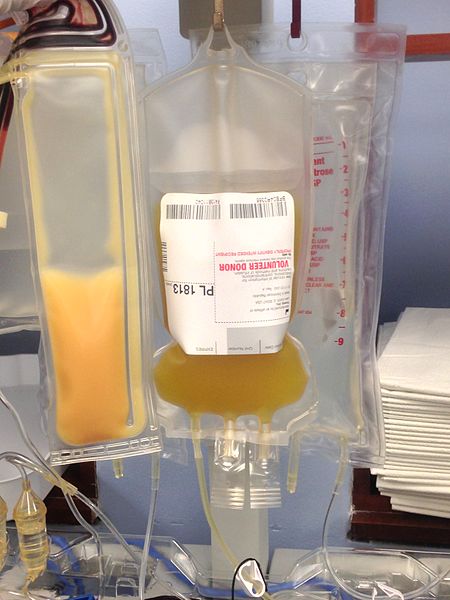 Understanding coagulation helps one understand the difference between plasma and serum. Plasma is the liquid portion of unclotted blood. Serum is the liquid portion of clotted blood. If a sample of blood hasn’t been allowed to clot, it still has available, yet-to-be-activated, clotting factors; therefore, it is plasma. If the sample has clotted, the clotting factors have been utilized, so it is serum. This is why a patient is given plasma, not serum. We don’t want to limit their ability to clot.
Understanding coagulation helps one understand the difference between plasma and serum. Plasma is the liquid portion of unclotted blood. Serum is the liquid portion of clotted blood. If a sample of blood hasn’t been allowed to clot, it still has available, yet-to-be-activated, clotting factors; therefore, it is plasma. If the sample has clotted, the clotting factors have been utilized, so it is serum. This is why a patient is given plasma, not serum. We don’t want to limit their ability to clot.
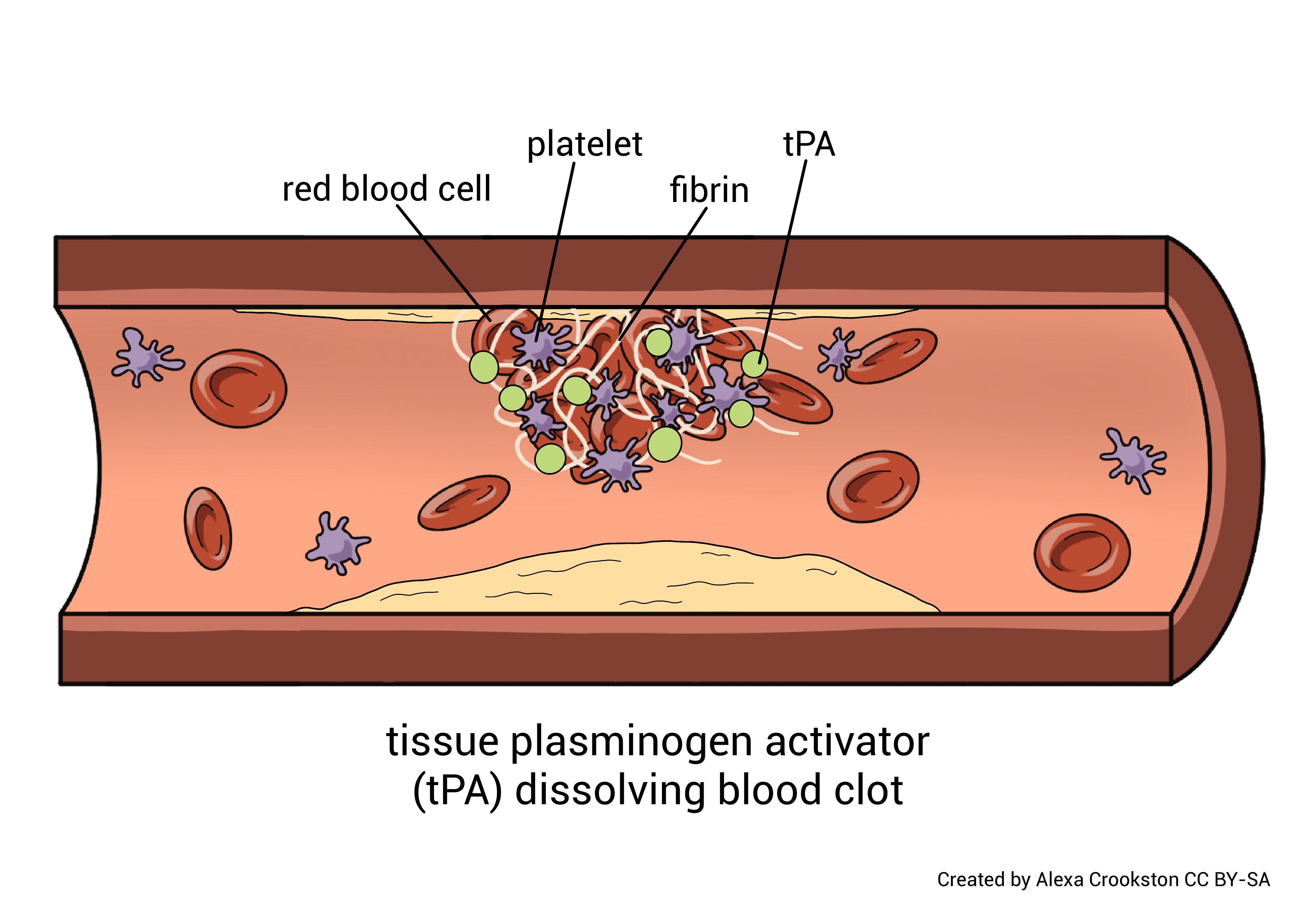
 Coagulation is not an infrequent event. People with minor trauma to tissue and blood vessels, clot multiple times per day, every day. If these clots were allowed to persist and accumulate, it would cause issues with blood flow. The body has a mechanism to dissolve these clots. It is called the fibrinolytic system and results in fibrinolysis (clot dissolution).
Coagulation is not an infrequent event. People with minor trauma to tissue and blood vessels, clot multiple times per day, every day. If these clots were allowed to persist and accumulate, it would cause issues with blood flow. The body has a mechanism to dissolve these clots. It is called the fibrinolytic system and results in fibrinolysis (clot dissolution).
As coagulation occurs, substances from both the tissue and the blood activate a protein called plasminogen to become plasmin. Plasmin is a very potent proteolytic enzyme. It dissolves the clot by digesting fibrin and interferes with new clot formation by inactivating fibrinogen, prothrombin, and other clotting factors.
Media Attributions
- U15-024 Platelets by Budding off from Megakaryocytes © パタゴニア is licensed under a CC BY-SA (Attribution ShareAlike) license
- U15-025 Hemostasis © Crookston, Alexa is licensed under a CC BY-SA (Attribution ShareAlike) license
- U15-026 Hemostasis © Betts, J. Gordon; Young, Kelly A.; Wise, James A.; Johnson, Eddie; Poe, Brandon; Kruse, Dean H. Korol, Oksana; Johnson, Jody E.; Womble, Mark & DeSaix, Peter adapted by Jim Hutchins is licensed under a CC BY (Attribution) license
- U15-027 Hemostasis © Betts, J. Gordon; Young, Kelly A.; Wise, James A.; Johnson, Eddie; Poe, Brandon; Kruse, Dean H. Korol, Oksana; Johnson, Jody E.; Womble, Mark & DeSaix, Peter adapted by Jim Hutchins is licensed under a CC BY (Attribution) license
- U15-028 Red Blood Cells In Clot © Fuzis is licensed under a CC BY-SA (Attribution ShareAlike) license
- U15-029 Plasma and platelet donation © Whoisjohngalt is licensed under a CC BY-SA (Attribution ShareAlike) license
- U15-030 Cutting Rope © Crookston, Alexa is licensed under a CC BY-SA (Attribution ShareAlike) license
- U15-031 tPA Dissolving Blood Clot © Crookston, Alexa is licensed under a CC BY-SA (Attribution ShareAlike) license

