Mechanisms of Air Movement
Objective 5
Define pulmonary ventilation, inspiration, and expiration. Define: Boyle’s Law. Explain the application of Boyle’s Law to inspiration and expiration. State the pressures in the structures of the respiratory system during inspiration and expiration.
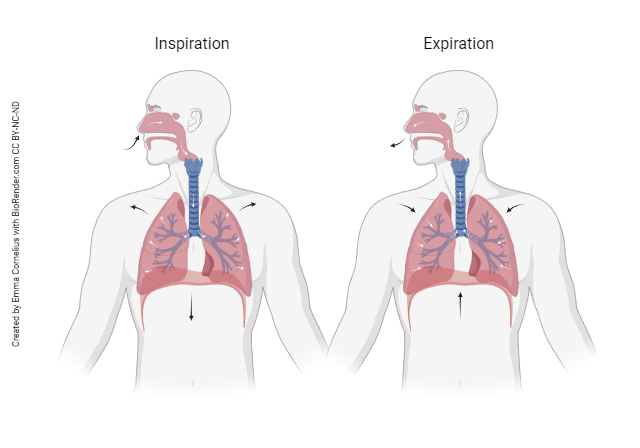
Let’s start by getting familiar with some more pulmonary terminology. Having a clear understand of what each word means will be important when describing and understanding the respiratory system.
Respiration: The process of gas exchange in the body.
Pulmonary Ventilation: The inhalation and exhalation of air. This involves the exchange of air between the atmosphere and the alveoli of the lungs.
Inspiration (inhalation): Movement of air into the lungs from the atmosphere.
-
- Active process requiring muscle action
Expiration (exhalation): Movement of air out of the lungs into the atmosphere.
-
- Passive process during quiet breathing due to the elastic recoil of the lungs
- Active (muscle help) during vigorous exercise or certain disease conditions causing difficult expiration (e.g. chronic obstructive pulmonary diseases)
The Kinetic Molecular Theory
Let’s shift our attention to two important physics laws and the principles behind these laws that will help us understand respiration. The idea that gas molecules are like a bunch of little billiard balls zipping around and colliding with each other is called by the rather fancy name The Kinetic Molecular Theory. There are five principles in the theory:
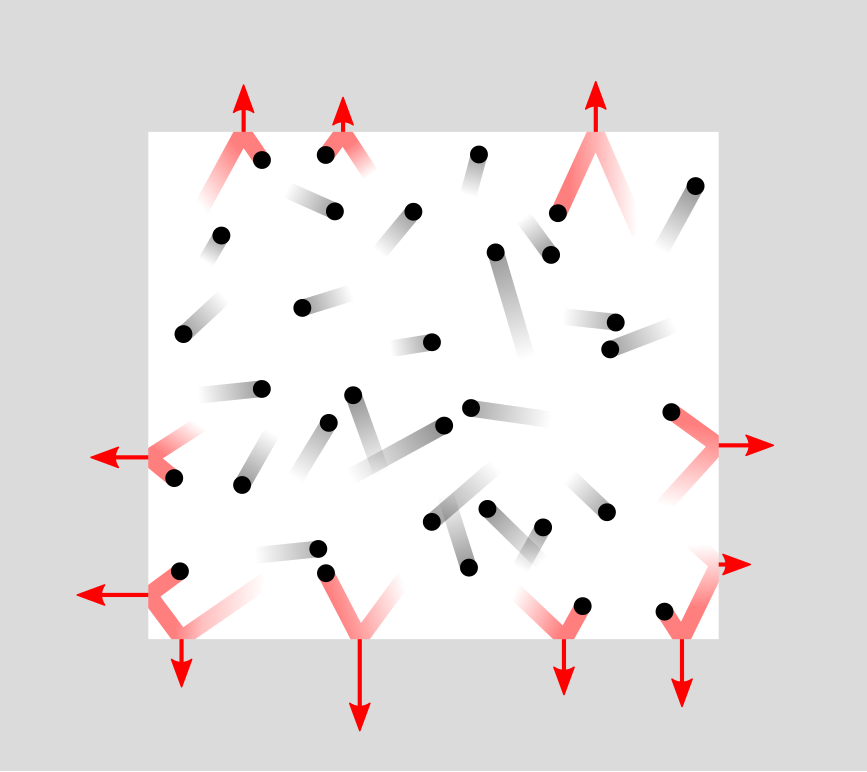 There is a lot more space between gas particles than the gas particles themselves occupy.
There is a lot more space between gas particles than the gas particles themselves occupy.- Particles move in a straight line until they collide. They move in different directions and have different speeds.
- The particles in a gas don’t interact with each other much, if at all.
- When particles collide, all the energy goes into bouncing, and none is absorbed by the particle.
- The average speed of the particles is related to the temperature.
In the 19th century, a number of scientists postulated gas laws. These laws were “absorbed” into the Kinetic Molecular Theory but we still learn them by the name of the person who stated them first.
Boyle’s Law
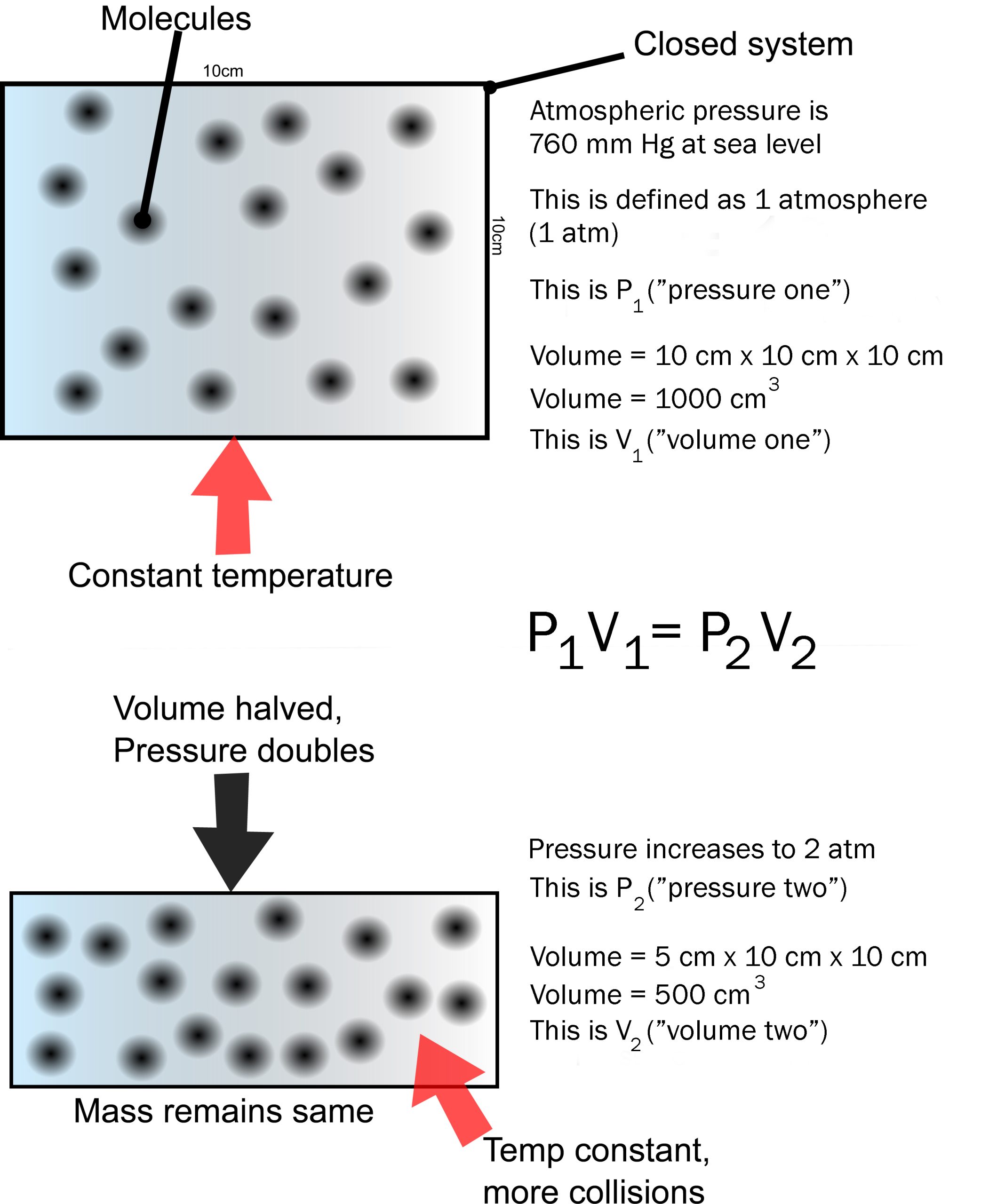
Pressure is the number of collisions with the wall of the container. Remember temperature is the average speed of the molecules.
Boyle’s Law says that pressure times volume is a constant at constant temperature. Human bodies are at a constant temperature of 37°C.
If volume goes down, pressure goes up. (If you press on a closed syringe, it’s volume decreases as you feel the pressure increase.)
If volume goes up, pressure goes down. (That is, there is more room between the molecules and fewer collisions.)
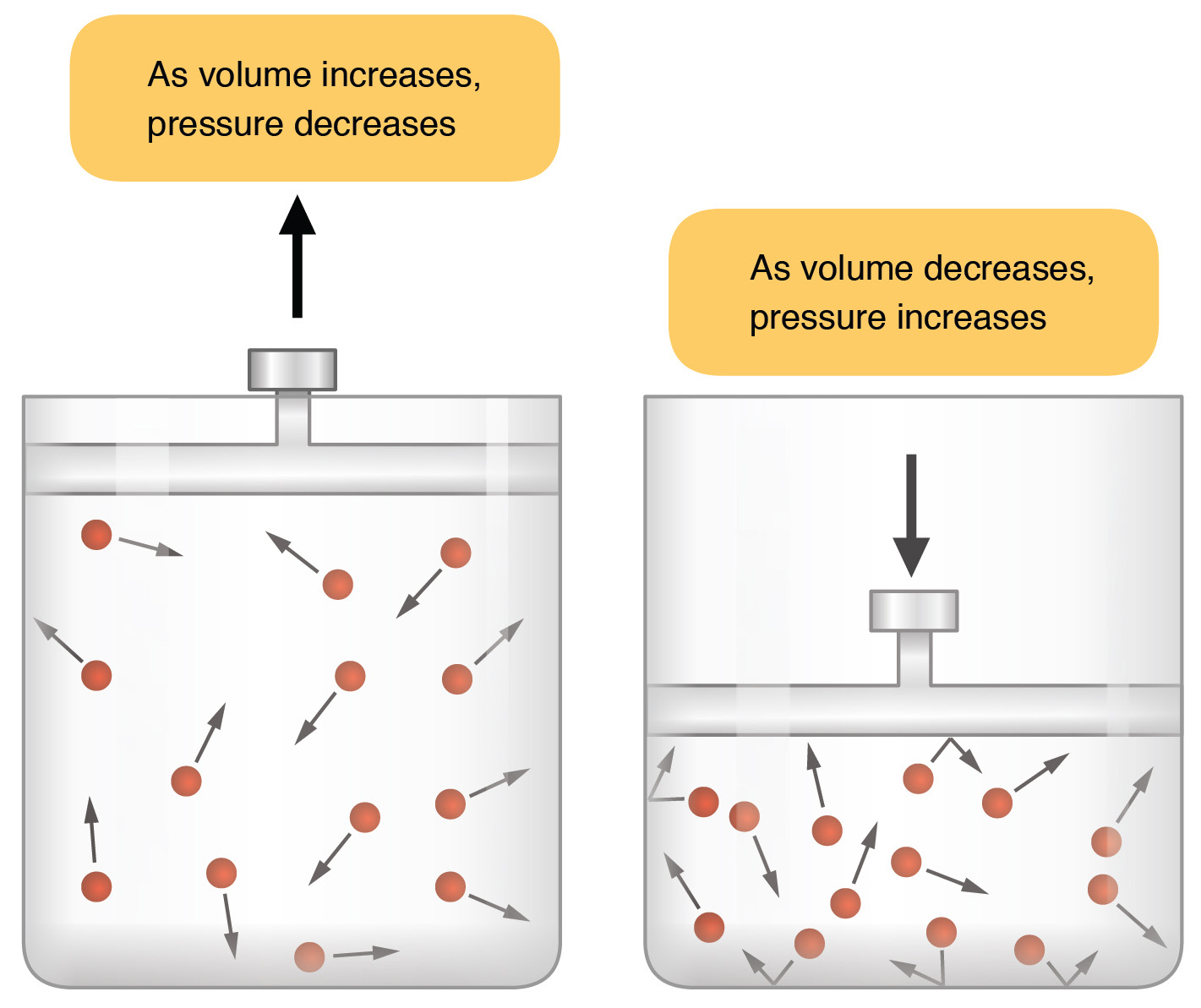
Boyle’s law has a direct application to the principles governing inspiration and expiration. During inspiration, contraction of the diaphragm and external intercostals muscles increase the volume of the thoracic cavity. As we just learned, as the volume increases, pressure decreases. The pressure in the thoracic cavity is now slightly less than atmospheric pressure. Recall that diffusion operates because molecules move from where they are at higher concentration to where they are at lower concentration. Because the molecules are at higher concentration when pressure increases, molecules move from regions of high pressure (high concentration) to regions of low pressure (low concentration).
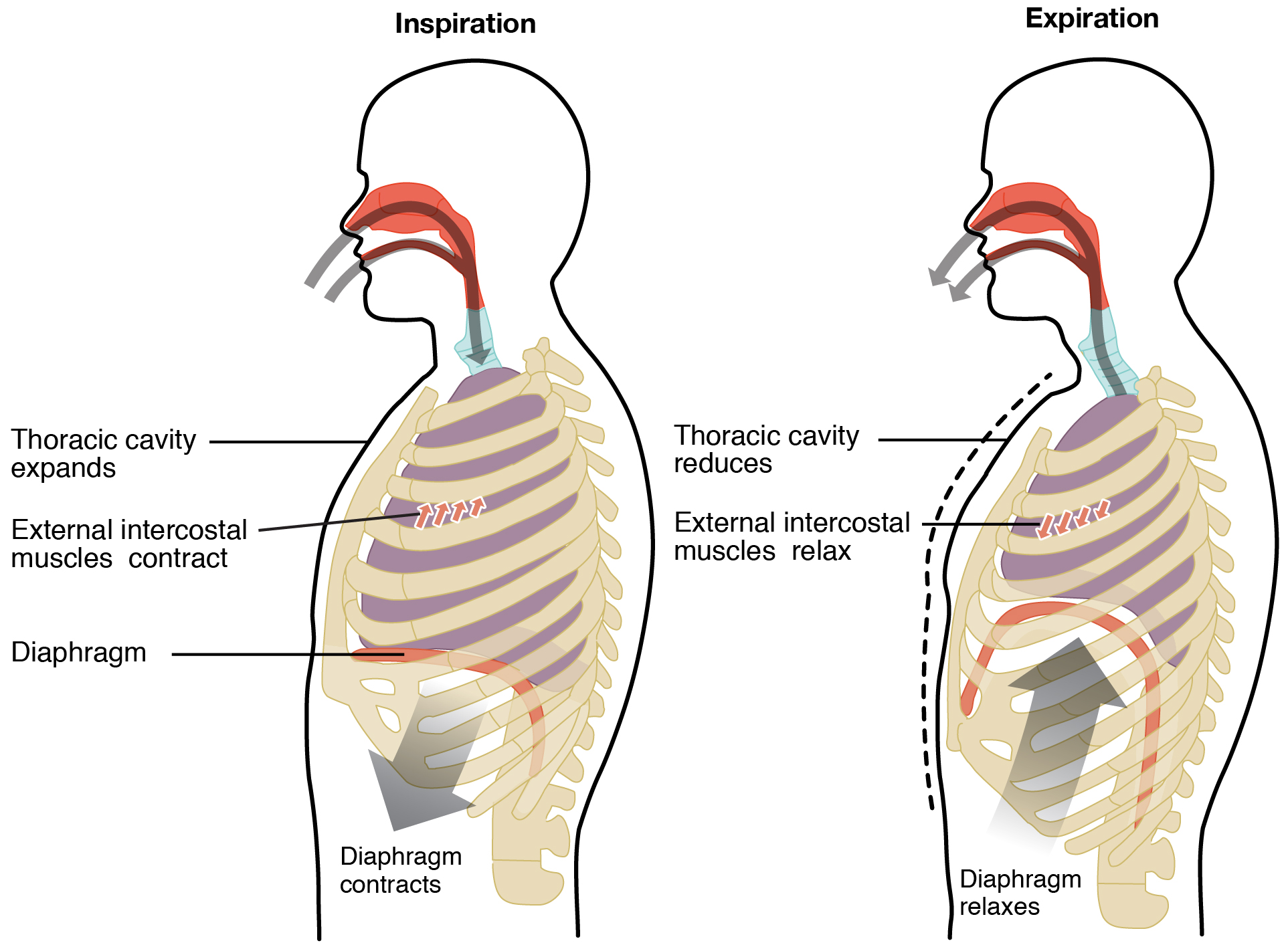
During deep, labored breathing, additional muscles are used to further enlarge the thoracic cavity. The accessory muscles include the sternocleidomastoid which elevates the sternum, the scalene muscles and pectoralis minor which elevate ribs.
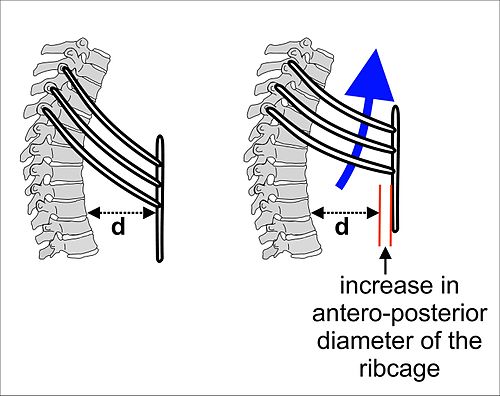
During inhalation the lifting of the sternum acts as the handle of a pump while the ribs elevate up and out like the handles of a bucket.
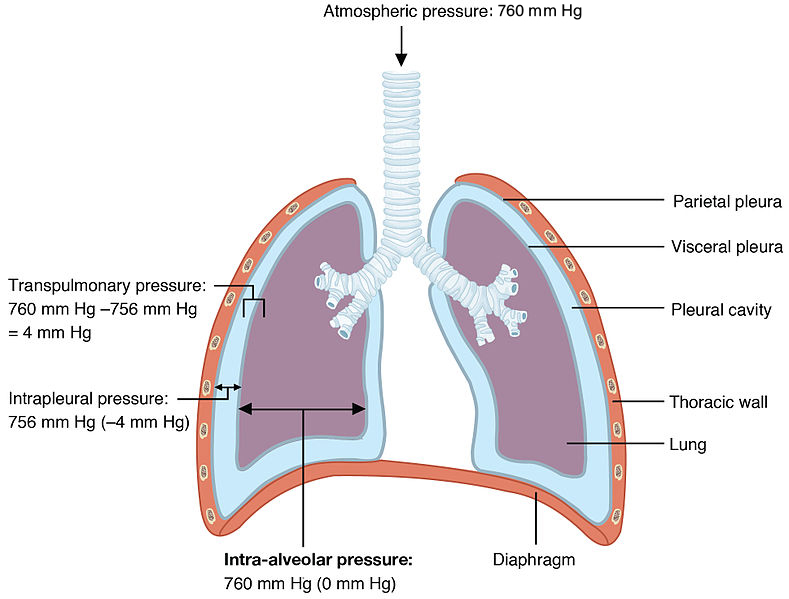
Exhalation is a passive process during quiet breathing. Elastic recoil of the chest wall and lungs causes the volume in the thoracic cavity to decrease. As the volume decreases, the pressure increases. Pressure is now higher in the thoracic cavity than atmospheric air and air flows out from high to low pressure.
During forced exhalation, abdominal muscles and the internal intercostals can contract and further decrease the volume of the thoracic cavity and increase the pressure.
Clinical Connection
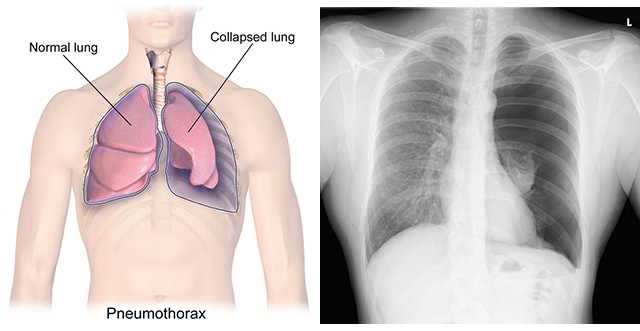
Pneumothorax
Air may leak into the pleural cavity from trauma to the lung or a spontaneous rupture of a bleb, a weak spot on the lung. Since the leak prevents a difference in pressure from developing, the lung does not fully inflate and a pneumothorax, or collapsed lung, may occur. Physicians treat a pneumothorax by placing a chest tube between the ribs in the wall of the thoracic cavity. This allows the air to flow out. The tube is then removed or sealed off and the hole in the chest wall repaired so the lungs can spontaneously re-inflate.
Media Attributions
- U17-028 Inspiration and Expiration © Cornelius, Emma is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- U17-029 Translational Motion © Greg L. is licensed under a Public Domain license
- U17-030 Pressure Exerted by Collisions © BECarlson is licensed under a CC BY-SA (Attribution ShareAlike) license
- U17-032 Boyles Law © Betts, J. Gordon; Young, Kelly A.; Wise, James A.; Johnson, Eddie; Poe, Brandon; Kruse, Dean H. Korol, Oksana; Johnson, Jody E.; Womble, Mark & DeSaix, Peter adapted by Jim Hutchins is licensed under a CC BY (Attribution) license
- U17-033 Pressures Movements Inspiration Expiration © Betts, J. Gordon; Young, Kelly A.; Wise, James A.; Johnson, Eddie; Poe, Brandon; Kruse, Dean H. Korol, Oksana; Johnson, Jody E.; Womble, Mark & DeSaix, Peter is licensed under a CC BY (Attribution) license
- U17-034 Sternum Pump Handle © Cruithne9 is licensed under a CC BY-SA (Attribution ShareAlike) license
- U17-035 Pressure Comparisons © Betts, J. Gordon; Young, Kelly A.; Wise, James A.; Johnson, Eddie; Poe, Brandon; Kruse, Dean H. Korol, Oksana; Johnson, Jody E.; Womble, Mark & DeSaix, Peter is licensed under a CC BY (Attribution) license
- U17-036a Composite Image Pneumothorax © BruceBlaus and Bickle, Ian adapted by Jim Hutchins is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license

