External and Internal Respiration
Objective 7
State Dalton’s Law and Henry’s Law. Explain how each is relevant to external and internal respiration. Using these laws, compare and contrast human physiology at normal atmospheric pressure and at high pressure. Describe, in detail, the process of external respiration and movement of gases across the alveolar-capillary (A-C) membrane and explain ventilation-perfusion coupling. Describe the process of internal respiration. Compare and contrast the processes of external and internal respiration. Compare and contrast the partial pressures of oxygen and carbon dioxide during the process of external and internal respiration.
External and internal respiration refer to the exchange of gases at the alveoli and at the tissues. These processes are explained by two physical laws: Dalton’s Law and Henry’s Law.
Dalton’s Law
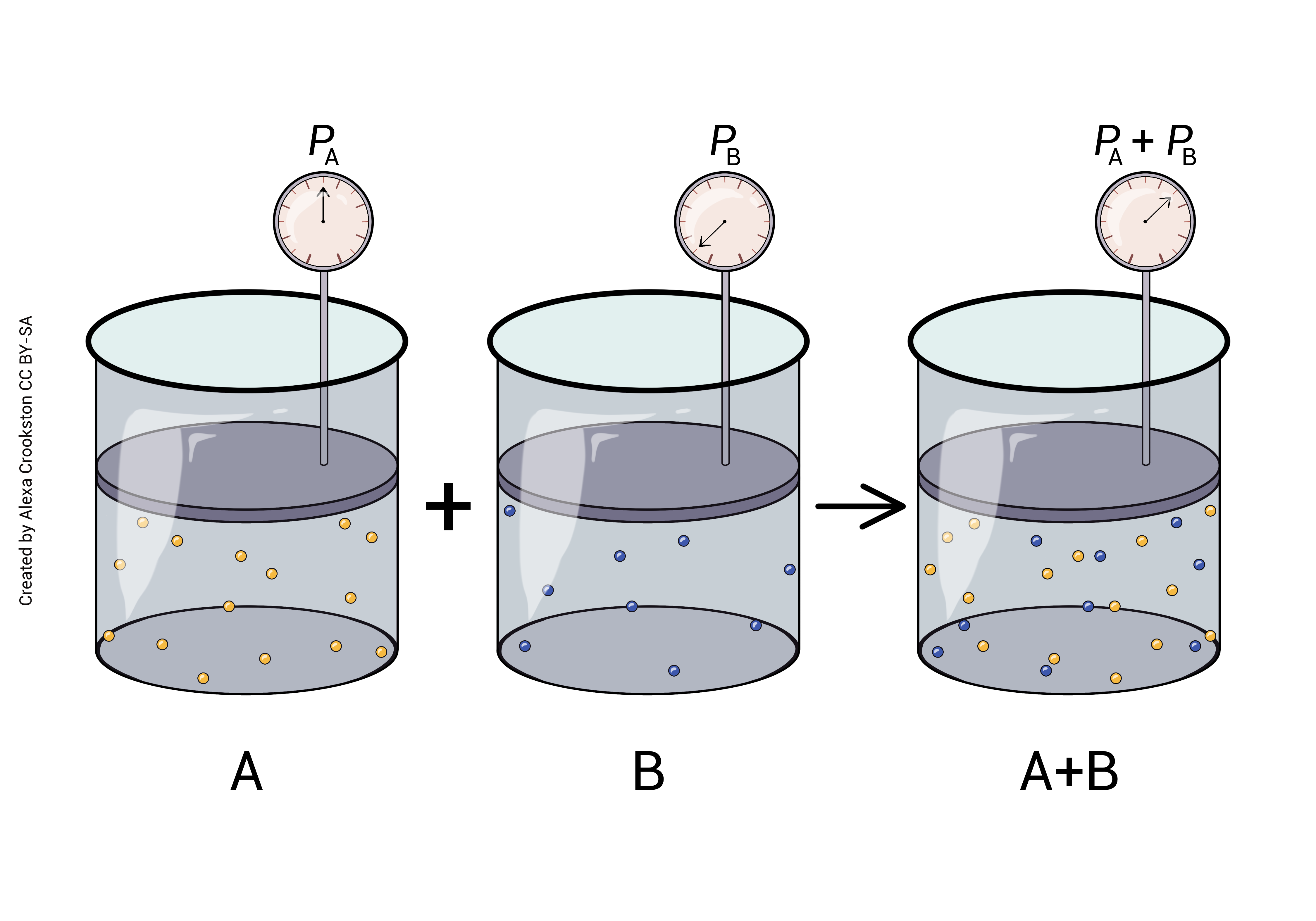
Dalton’s Law says that the particles in a gas don’t care about each other. In a mixture of different gases (gas A and gas B and gas C), the pressure due to gas A is exactly equal to its proportion in the mixture.
Atmospheric pressure is defined as 760 mm Hg at sea level. This is also called a pressure of 1 atmosphere (1 atm = 760 mm Hg). This is the pressure (collisions) created by a column of air 10 miles (16 km) high. These collisions, this pressure, can lift a column of mercury (Hg) 760 mm. When you listen to the weather report, they call this "30 inches" (30 inches Hg = 760 mm Hg). Remember from Unit 16 that systolic blood pressure is about 1/6 atm or 120 mm Hg.
Because 21% of the atmosphere is oxygen, 21% of the collisions (pressure) are due to oxygen. We call this the partial pressure of oxygen.
Henry’s Law
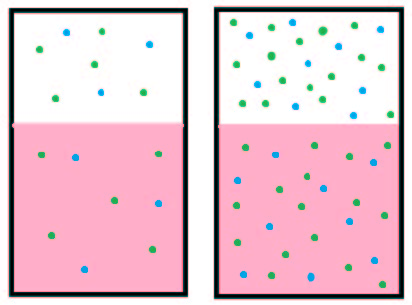
Henry’s Law says the amount of a gas that is dissolved in a liquid is directly proportional to the partial pressure of the gas. In the body much more CO2 is dissolved in blood plasma than O2 because it is 24x more soluble than oxygen. A hyperbaric chamber increases the atmospheric pressure of oxygen, and more dissolves in solution (the blood).
When Olympians came to Salt Lake City to compete in the 2002 Winter Olympics, they had to contend with the high altitude. Atmospheric pressure decreases as we ascend in altitude because the column of air above your head is 1500 m (5000 ft) shorter. Henry’s Law states that if the atmospheric pressure of oxygen is lower, less will dissolve in solution. Altitude sickness may occur because of the lack of oxygen dissolved in the blood. Ultimately pulmonary vessels may vasoconstrict increasing pulmonary pressure. Fluid may be pushed out of the vessels leading to the serious condition of pulmonary edema. Lack of oxygen to the brain may lead to cerebral edema and subsequent death.
The opposite condition may occur with scuba divers. For every 30 feet that a diver descends, atmospheric pressure increases by 1 atm (760 mmHg). A much larger amount of nitrogen than normal is now dissolved in the blood because of the high pressure. If a diver ascends too rapidly, nitrogen may come out of solution leading to joint and lung damage, a painful condition called “the bends”.
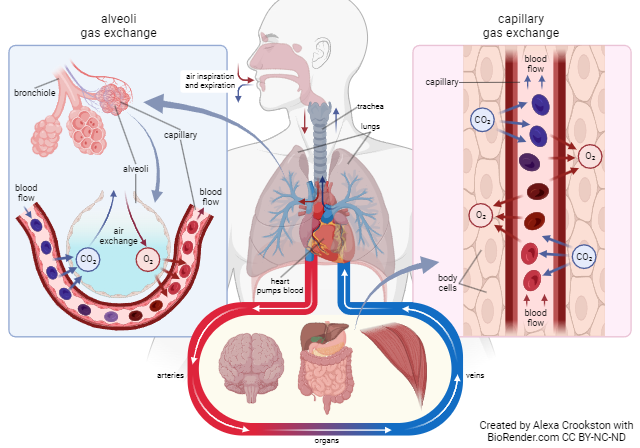
External Respiration
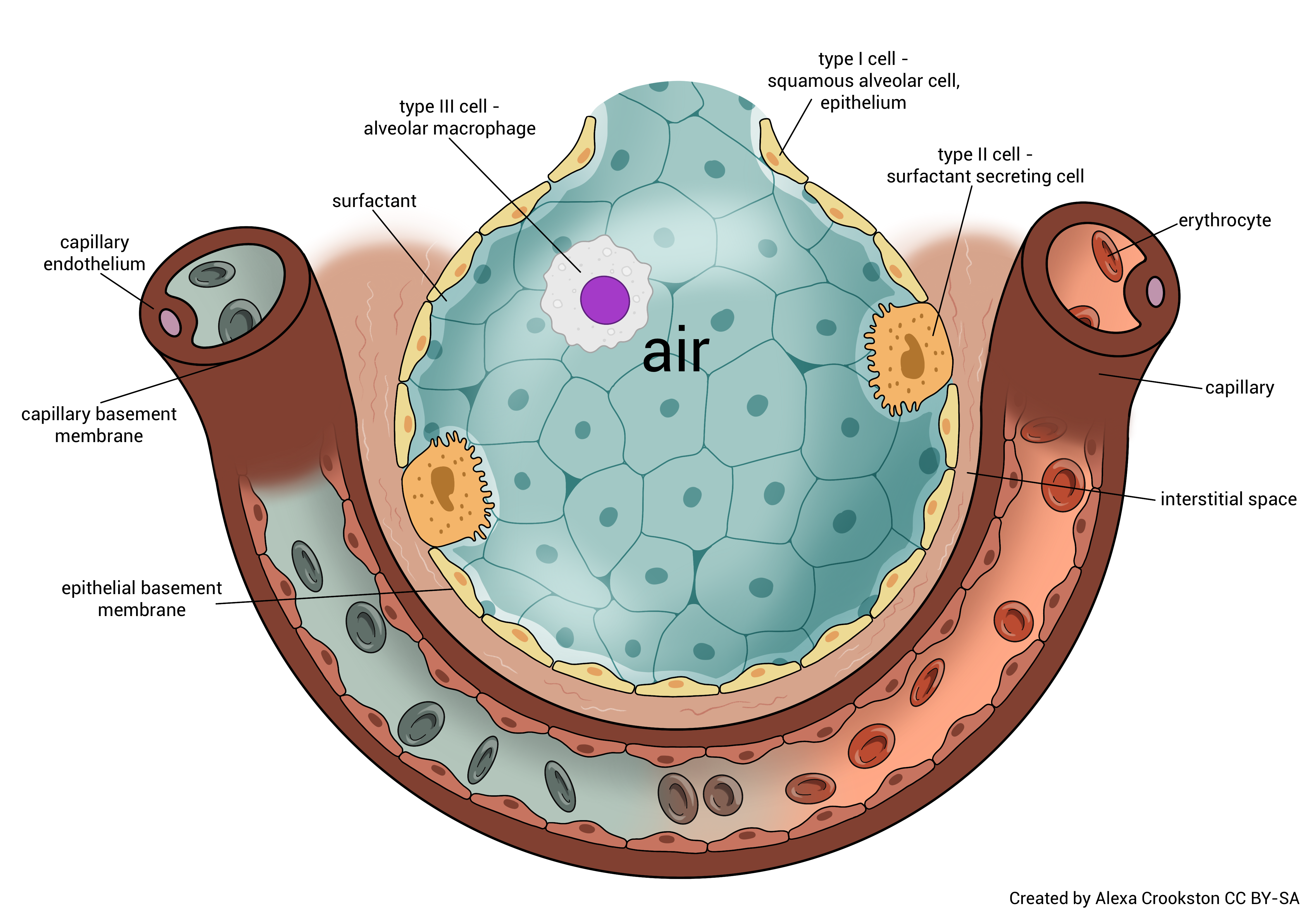
External respiration occurs at the alveolocapillary membrane. Oxygen diffuses from the alveoli of the lungs into blood in the pulmonary capillaries. CO2 diffuses from the blood in the pulmonary capillaries into the alveoli of the lungs. (Remember that the tissues created CO2 as a by-product of cellular respiration, and placed it in the blood on the venule side of the capillary bed in the tissues.)
The combination of these two diffusion events, oxygen moving from where it is at high concentration (alveolar air spaces) to where it is at low concentration (pulmonary capillaries) and CO2 moving from where it is at high concentration (pulmonary capillaries) to where it is at low concentration (alveolar air spaces) is called external respiration.
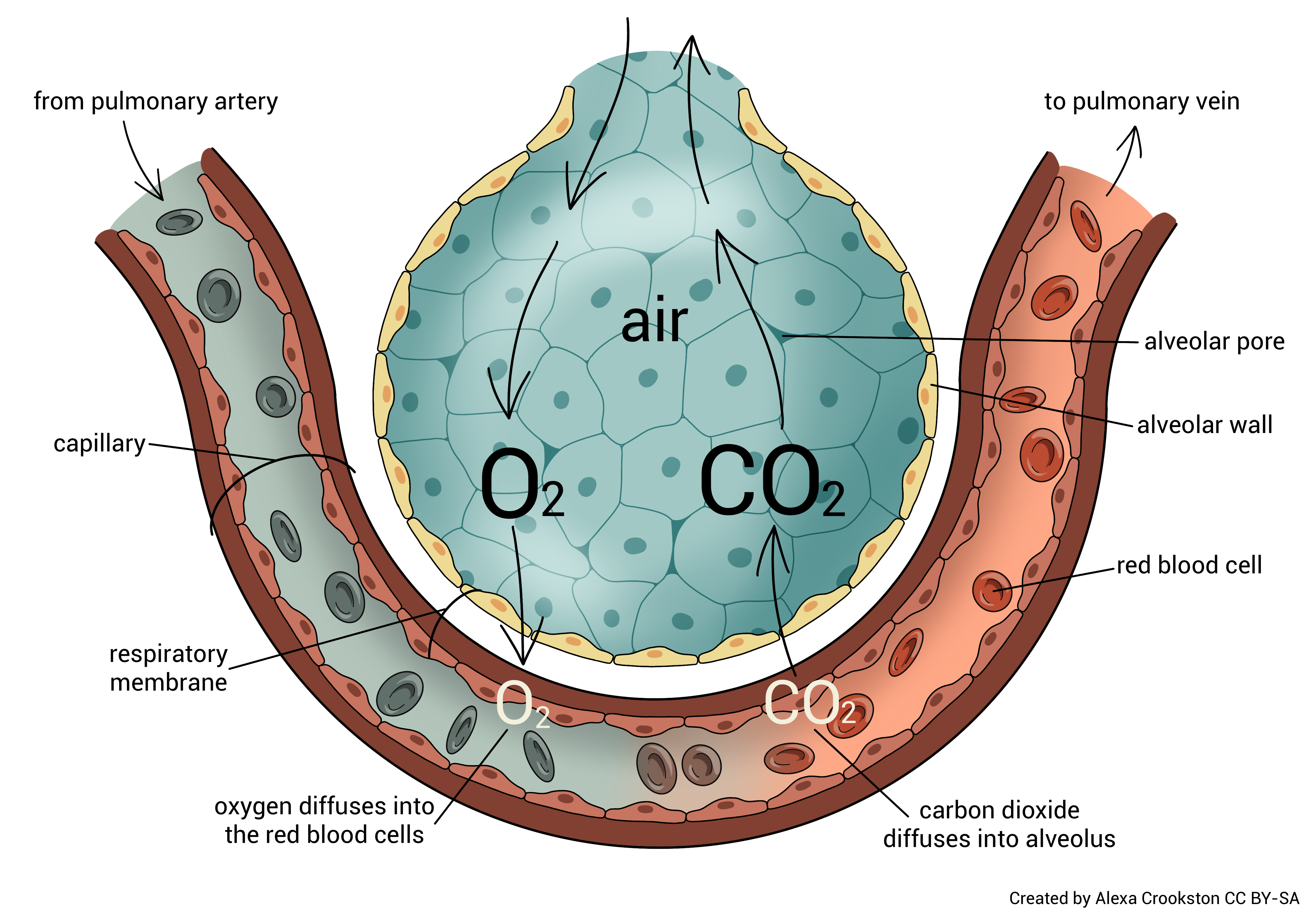
While the alveolocapillary membrane is very thin (about 1/2000 of a millimeter or 0.5 µm) an oxygen molecule must still pass through three layers. The alveolar basement membrane and the capillary basement membrane are fused into a single layer, which shortens the journey. Oxygen must pass through the type I alveolar cell, the fused basement membranes, and the endothelial cell of the capillary. The entire distance traveled is about 0.5 μm.
Waste-laden blood from the tissues returns to the heart and then enters the lungs via the pulmonary arteries and arterioles. CO2 diffuses across the alveolar-capillary membrane (opposite of how the oxygen molecule moves) and is exhaled.
O2 from the atmosphere diffuses from the alveoli to the blood. This now oxygen-rich blood returns to the left atrium of the heart via the pulmonary venules and veins. It is then pumped out to the tissues of the body.
Remember that gases diffuse independently of one another from an area of high concentration (partial pressure) to an area of low concentration (partial pressure). CO2 and O2 are simply diffusing down their concentration gradients
 An important concept to discuss with external respiration is ventilation-perfusion coupling. Let’s start with some definitions:
An important concept to discuss with external respiration is ventilation-perfusion coupling. Let’s start with some definitions:
Pulmonary ventilation ([latex]\dot{V}[/latex]): is the amount of air entering the lungs each minute. (The “V” is for ventilation; the dot indicates per minute.)
Alveolar ventilation ([latex]\dot{V_A}[/latex])is the amount of air entering the alveoli each minute. If air enters your lungs, but does not enter the alveoli, then it can't cross the A-C membrane and its gases cannot be absorbed into the blood.
Perfusion ([latex]\dot{Q}[/latex]) is the amount of blood that flows through the pulmonary capillaries each minute.
A ratio of ventilation to perfusion (VA/Q) [latex]\frac{\dot{V_A}}{\dot{Q}}[/latex] can be calculated and is normally about 1. This means that ventilation (air flow) and perfusion (blood flow) are equally matched. In healthy individuals, [latex]\dot{V_A}[/latex] is about 4.5 L/min and [latex]\dot{Q}[/latex] is about 5.0 L/min. If no air enters the lungs the [latex]\frac{\dot{V_A}}{\dot{Q}}[/latex] is zero, meaning blood is flowing but no gas exchange takes place because no air is entering the alveoli. If air enters the lungs but blood is not flowing then [latex]\frac{\dot{V_A}}{\dot{Q}}[/latex] is very high, approaching infinity.
The [latex]\frac{\dot{V_A}}{\dot{Q}}[/latex] ratio is changed in disease states that affect oxygen flow or blood flow. For example, if a patient has difficulty breathing, oxygen flow decreases and the ratio is disrupted (less air entering the lung but normal blood flow). If a patient has a blood clot in the lung, blood does not flow and the ratio is again disrupted (normal air intake but less or absent blood flow). If the ventilation/perfusion ratio is disrupted, inadequate exchange of oxygen and carbon dioxide occurs.
Under hypoxic conditions (low amounts of oxygen) pulmonary blood vessels constrict. This constriction causes blood to move (or shunt) from areas of low oxygen in the lungs to areas of high oxygen. Blood flow is greatest, therefore, in alveoli that have the greatest amount of oxygen flow. This matching of blood flow to oxygen is termed ventilation-perfusion coupling.
Internal Respiration
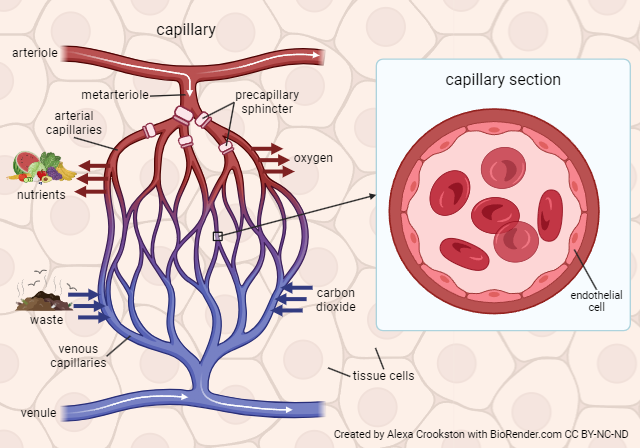
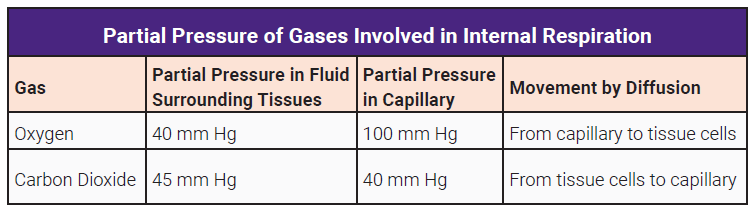
Internal respiration is the exchange of O2 and CO2 between systemic capillaries and tissue cells. This exchange occurs in tissues throughout the body. Gases again diffuse from high to low pressure. The tissues are actively using oxygen for metabolism. The partial pressure of oxygen is lower in the tissues and higher in the blood therefore oxygen diffuses into the tissues. CO2 is a waste product of metabolism. Its partial pressure is higher in the tissues than the blood so it diffuses from high to low, leaving the tissues and entering the bloodstream.
Comparing Internal & External Respiration
In summary, external respiration is the exchange of gases between the pulmonary capillaries and the alveoli which contain atmospheric air. CO2 diffuses from the capillaries into the alveoli; O2 diffuses from the alveoli into the pulmonary capillaries.
Internal respiration is the exchange of gases between the tissues and the systemic capillaries. Oxygen diffuses into the tissues; carbon dioxide diffuses from the tissues into the bloodstream.
Now let’s put it all together, since these two processes are seemingly one continuous circuit.
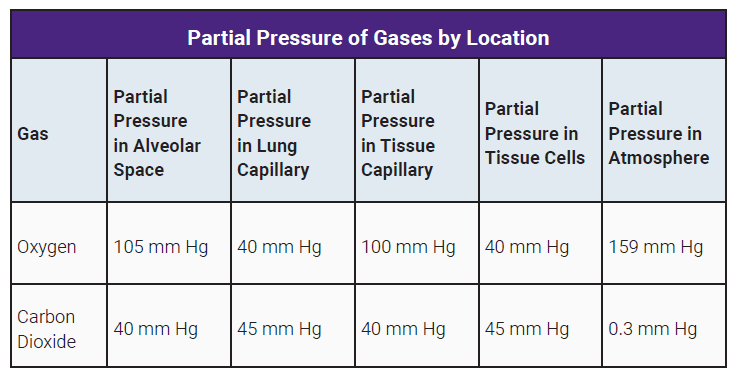
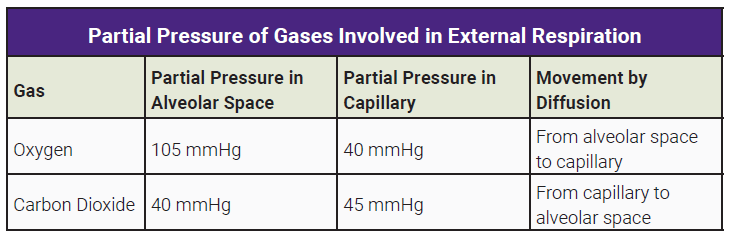
The PO2 is highest in the alveoli where it’s about 105 mm Hg.
The pressure drops slightly as blood enters the right atrium (100 mm Hg).
The PO2 in the tissues is only about 40 mm Hg. Thus, O2 flows from the higher pressure in the systemic capillaries into the tissues. As blood leaves the tissues, the PO2 drops to approximate that of the tissues, about 40 mm Hg.
At the same time, CO2 diffuses in the opposite direction. CO2 is highest in the tissues and in the blood returning to the right atrium and the lungs via the pulmonary arteries (PCO2 45 mm Hg).
Hence, CO2 diffuses into the alveoli where the pressure is close to atmospheric CO2 which is less than 1 mm Hg. CO2 leaving the lungs and in the systemic circulation traveling to the tissues has a partial pressure of 40 mm Hg. This is lower than the tissues (PCO2 45 mm Hg), thus, CO2 diffuses from the tissues to the systemic capillaries.
Media Attributions
- U17-040 Partial Pressure © Crookston, Alexa is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- U17-041 Partial Pressures Table © Burr, Justin is licensed under a CC BY-SA (Attribution ShareAlike) license
- U17-042 Henry’s Law © Crookston, Alexa is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- U17-044 Alveolus © Crookston, Alexa is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- U17-043_External Respiration © Crookston, Alexa is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- U17-045 Partial Pressures in External Respiration Table © Burr, Justin is licensed under a CC BY-SA (Attribution ShareAlike) license
- U17-028 Inspiration and Expiration © Cornelius, Emma is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- U17-047 Capillary © Crookston, Alexa is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- U17-048 Partial Pressures in Internal Respiration Table © Burr, Justin is licensed under a CC BY-SA (Attribution ShareAlike) license
- U17-049 Respiration © Crookston, Alexa is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license

