Electrolytes and Buffers
Objective 10
Compare the electrolyte and protein anion concentrations in plasma, interstitial fluid and intracellular fluid. Characterize the role of buffers, ventilation, and renal function in maintaining acid-base homeostasis.
Electrolytes & Protein
In addition to maintaining appropriate levels of water in the right places, the cells and tissues of the body expend energy to conserve necessary concentrations of electrolytes and proteins.
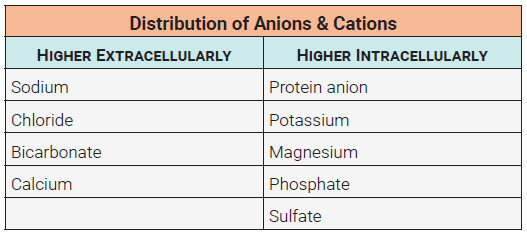
Although all of the electrolytes found in the body perform critical functions, their distribution varies significantly. Of the cations, sodium is by far the most abundant. Of the anions, chloride, bicarbonate, and negatively charged proteins top the list.
These concentrations are required to maintain the water balance previously mentioned as well as for a number of critical cell functions, like creating action potentials, moving substances in and out of cells, and creating body movements. Changes in solute concentrations can have detrimental effects on the body, so different systems work together to keep the body in homeostasis.
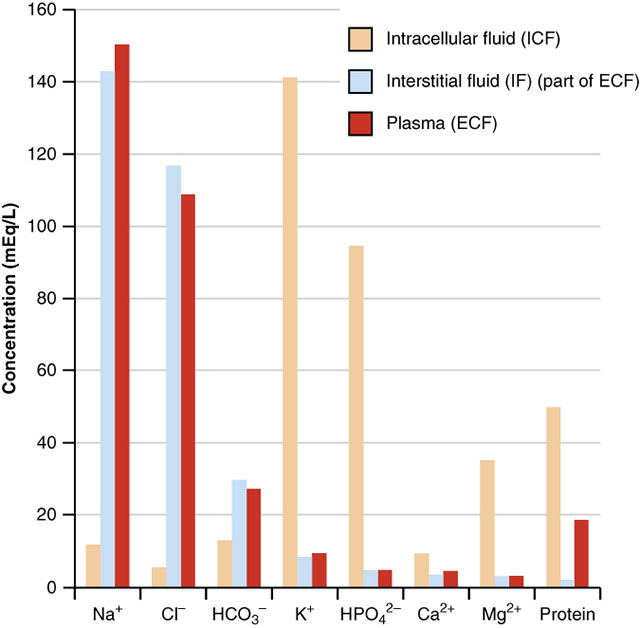
Active and passive transport mechanisms are used to establish solute gradients across cell membranes. The result of these movements is an intracellular environment that is typically higher in proteins, potassium, magnesium, phosphate, and sulfate ions. In the fluid surrounding cells, we would expect to see higher concentrations of sodium, chloride, bicarbonate, and calcium. During normal cell processes these relative concentrations will change, but are quickly reestablished.
pH & Buffers
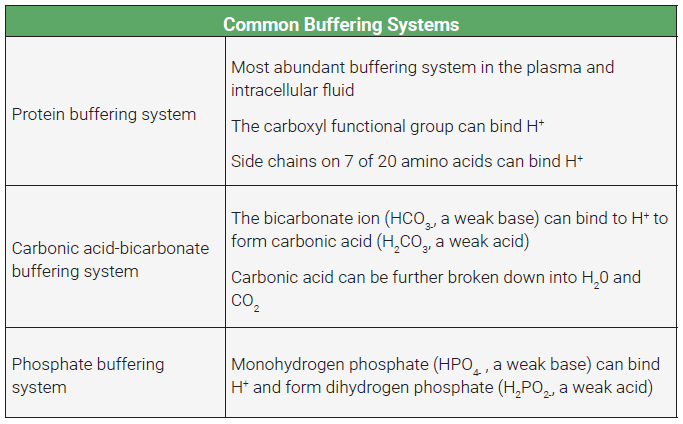
A solution is very acidic if it contains large numbers of free H+ ions. That doesn’t mean it simply contains the element hydrogen; it must contain free hydrogen ions, which are really just protons, if you think about it. If a molecule can temporarily bind H+ and, therefore, reduce the number that are free, it can reduce the acidity (increase the pH) of a solution. This is what a buffer does. It doesn’t remove the H+ ions from the body, it simply binds to them.
Respiratory & Renal Control of pH
As you may recall from Unit 17, changes in rate and depth of ventilation can alter the blood pH. As CO2 increases, so do H+ ions, and the pH drops, making the body more acidic. Any condition that decreases a person’s ability to breathe adequately can cause this. These conditions include emphysema, asthma, and drugs that suppress breathing.
If CO2 decreases, the H+ concentration likewise drops and the body becomes too alkaline. Conditions such as hyperventilation and anxiety can cause this.
The changes in pH can take place in minutes. That enables the respiratory system to be a quick fixer of pH problems in the body. It is also the reason why problems with the respiratory system can quickly lead to dangerous pH imbalances.
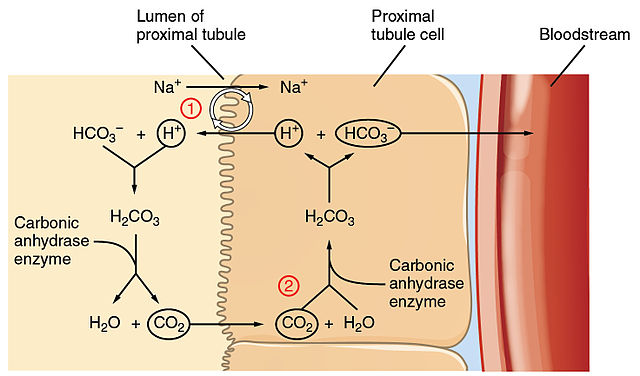
The renal system plays an important role in pH balance as well, however the effects are a little slower and often less dramatic than that of the respiratory system. The tubules of the nephron can reabsorb H+ ions or secrete them into filtrate. By reabsorbing H+ ions, the kidneys decrease blood pH. If the blood is too acidic, the kidneys can secrete the excess H+ ions into the filtrate. In a similar way, the kidneys can control HCO3– ions to help maintain normal pH. Tubular cells do not absorb HCO3– ions, rather they can produce them and then use cell metabolism to use facilitate the carbonic acid-bicarbonate buffer system. This is why you may hear it said that bicarbonate is conserved, not reabsorbed. As the kidneys work to maintain normal blood pH, the excreted bicarbonate and H+ ions change the urine pH, but that doesn’t matter as urine is waste.
Media Attributions
- U19-041 Table Distribution of Anions and Cations © Price, Travis is licensed under a CC BY-SA (Attribution ShareAlike) license
- U19-042 Concentration of Elements in Body Fluids © Betts, J. Gordon; Young, Kelly A.; Wise, James A.; Johnson, Eddie; Poe, Brandon; Kruse, Dean H. Korol, Oksana; Johnson, Jody E.; Womble, Mark & DeSaix, Peter is licensed under a CC BY (Attribution) license
- U19-043 Table of Common Buffer Systems © Price, Travis is licensed under a CC BY-SA (Attribution ShareAlike) license
- U19-044 Conservation of Bicarbonate in Kidney © Betts, J. Gordon; Young, Kelly A.; Wise, James A.; Johnson, Eddie; Poe, Brandon; Kruse, Dean H. Korol, Oksana; Johnson, Jody E.; Womble, Mark & DeSaix, Peter is licensed under a CC BY (Attribution) license

