Conception | Embryonic and Fetal Periods
Objective 10
Explain the process of conception, including coitus, sperm capacitation, slow block to polyspermy, and fertilization. Describe embryonic events from fertilization to gastrulation and the development of extra-embryonic membranes. Describe fetal events from gastrulation to organogenesis.
Conception
Conception begins with sexual intercourse, or coitus. The male’s penis is inserted into the female’s vagina. When ejaculation occurs, about 300 million spermatozoa are released into the female reproductive tract. These sperm must negotiate the route from the vagina to the cervix, through the uterus, and into both uterine tubes (they don’t know which one might contain the secondary oocyte). Only a few hundred, at most, will make it into each uterine tube. Amazingly, some sperm complete the entire journey in just 10-15 minutes.
Sperm Capacitation
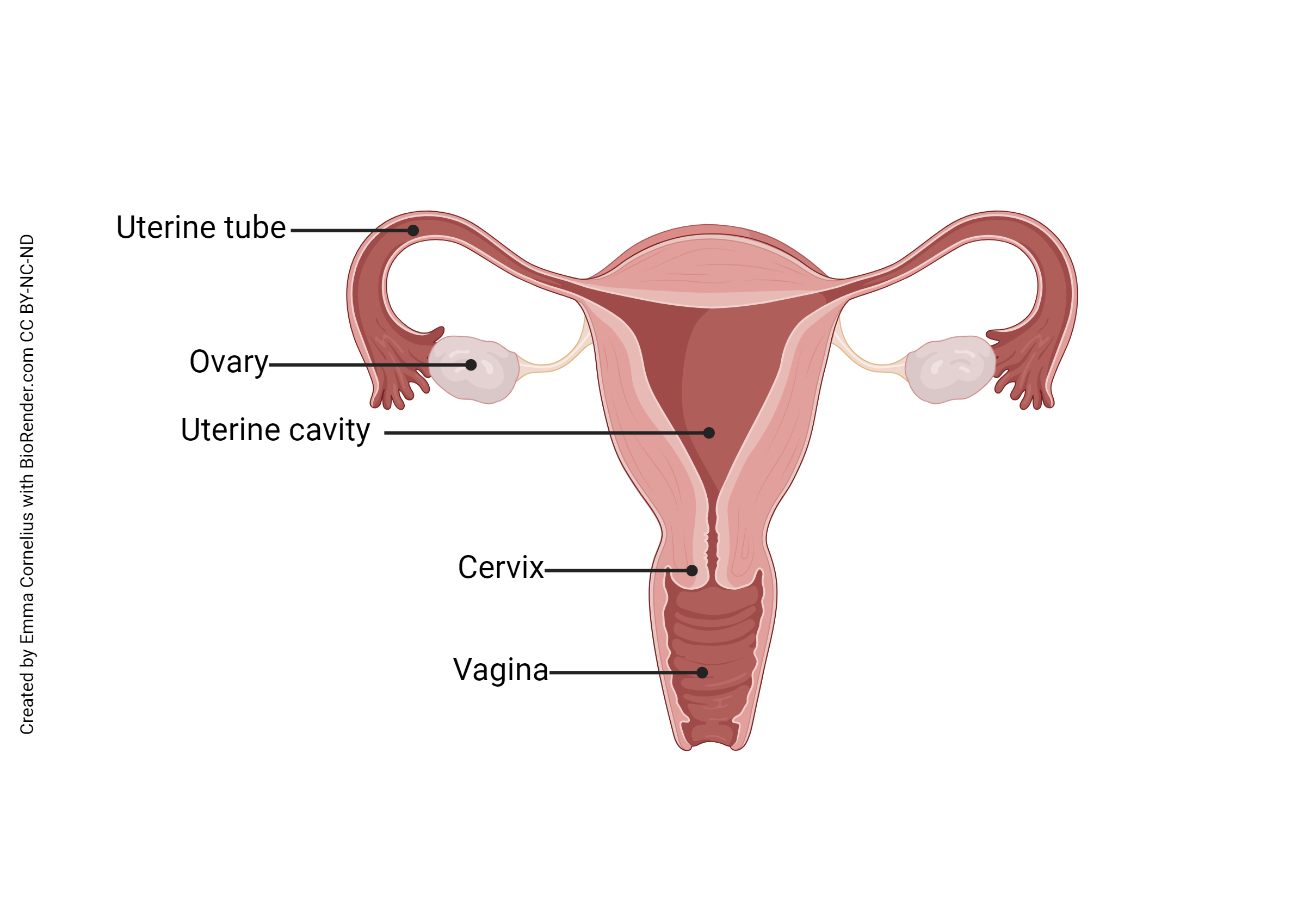
In the uterine tube, a process called sperm capacitation takes place. The word “capacitation” means “to make capable” or “enable.” In this case, those few spermatozoa that have made it this far are made capable of penetrating a secondary oocyte. Their flagella beat more strongly and their acrosomes lose cholesterol, proteins, and glycoproteins, and activate profilactin (a precursor protein) to become actin, all in preparation to fuse with the oocyte plasma membrane.
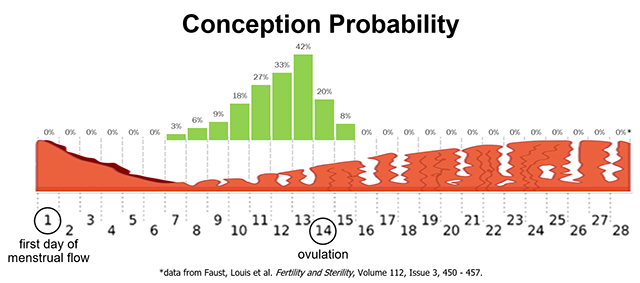
Fertilization (conception) occurs when a single spermatozoon penetrates the secondary oocyte, causing it to complete meiosis II and become an ovum. This usually occurs in the first third of the uterine tube, near the ovary. Spermatozoa can survive in the female reproductive tract up to seven days after ejaculation. The unfertilized secondary oocyte survives up to two days after ovulation. This gives a theoretical window of about five days during the female reproductive cycle when conception can occur. As you can see from the graph in the image, that window is actually a bit wider, about nine days. The probability of conception from a single act of intercourse without birth control ranges from zero during menstruation to a peak of about 40% the day before ovulation (Day 13), back to zero the second day after ovulation (Day 16).
Blocks to Polyspermy
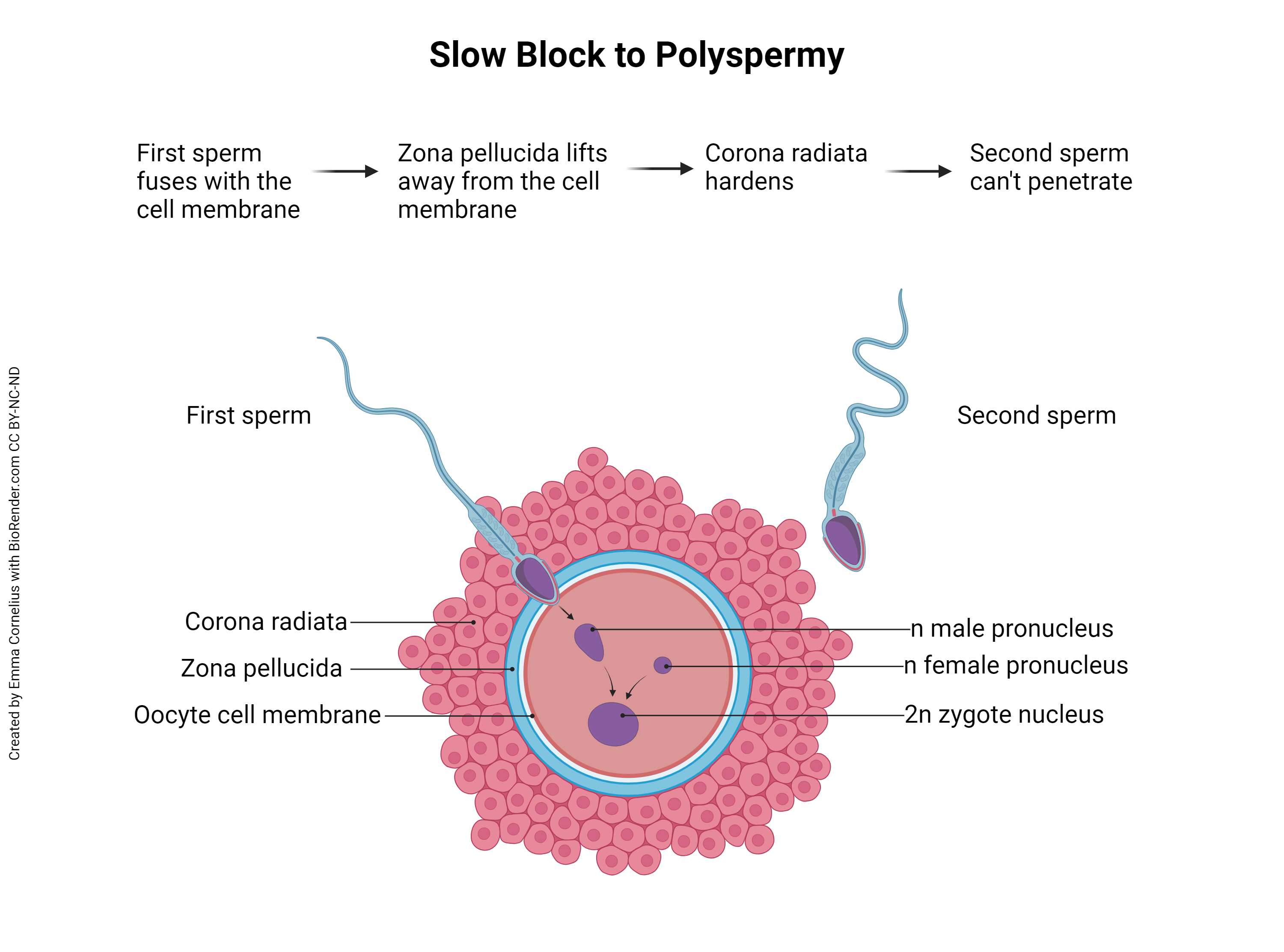
Note the first sentence of the previous paragraph, “Fertilization occurs when a single spermatozoon penetrates the secondary oocyte…” This is important. Remember that the sperm is haploid and the ovum is haploid, and together they form a diploid zygote. What would happen if two or more sperm were able to penetrate and fertilize the secondary oocyte? This is called polyspermy and, instead of haploid (23C) plus haploid (23C) equals diploid (23C + 23C = 46C), the resulting zygote would be haploid times three equals triploid (69C) or haploid times four equals tetraploid (92C), etc. In other words, there would be three or four or more copies of each DNA molecule in the nucleus of the new organism. Unfortunately, any ploidy number greater than diploid (ie, triploidy, tetraploidy, etc) is incompatible with human life. To prevent this, the ovum and sperm must work together to prevent polyspermy.
There are at least two different methods to prevent polyspermy — the fast block to polyspermy and the slow block to polyspermy. The fast block occurs within a tenth of a second of sperm penetration and is seen in many animal species, but doesn’t seem to function in mammals (including humans). Mammals, instead, employ the slow block to polyspermy. Back when the secondary oocyte was released from the mature follicle (ovulation), it carried with it a surrounding layer of granulosa cells called the corona radiata (Latin, “radiant crown”). A thick, clear layer, the zona pellucida (Latin, “transparent zone”), also surrounds the oocyte, just inside the corona radiata. The first spermatozoon to penetrate the corona radiata and zona pellucida will fuse with the oocyte cell membrane. This fusion triggers changes to the cell membrane which prevent fusion by other sperm. Additionally, the zona pellucida lifts away from the cell membrane and the corona radiata hardens, both of which prevent additional sperm from reaching the oocyte cell membrane.
Remember that when the secondary oocyte is penetrated by a sperm, it rapidly completes meiosis II, discards the second polar body, and becomes an ovum. The ovum’s membrane-enclosed DNA is called the female pronucleus. Also now inside the ovum is the sperm’s membrane-bound DNA (it was endocytosed as the sperm fused with the oocyte cell membrane), called the male pronucleus. When the two pronuclei join to form a single nucleus inside the ovum, fertilization has occurred. There is some disagreement regarding the definition of conception – some equate conception with fertilization, while others define conception as the time of implantation (about six days after fertilization). Fertilization, on the other hand, is an unambiguous term referring to union of sperm and egg DNA. When that occurs, the fertilized ovum becomes a zygote and the developmental clock begins.
Clinical Connection: Ectopic Pregnancy
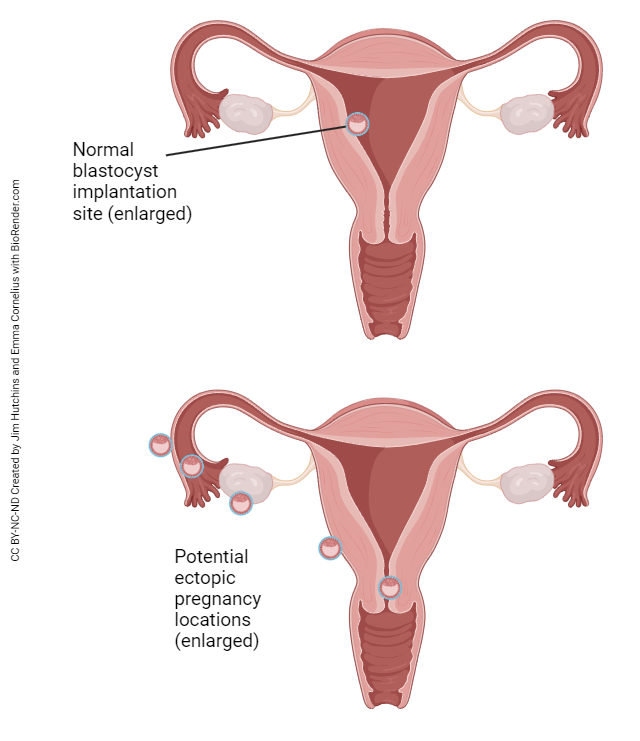 The connection between the surface of the ovary and the uterine tube is not fully enclosed. At ovulation, the released oocyte must find its way into the uterine tube with the help of the fimbriae (finger-like projections from the open end of the uterine tube that move, creating a current to pull the oocyte into the uterine tube). From time to time, an oocyte does not make it into the uterine tube and ends up in the peritoneal cavity. Sperm can also find their way through the uterine tube and into the peritoneal cavity. If the egg and sperm meet and fertilization occurs, the embryo will attempt to implant wherever it can – the ovarian surface, the peritoneal surface of the uterus, the outside surface of the uterine tube, somewhere else in the abdomen. Obviously, childbirth can never occur under these circumstances. The resulting pregnancy is called an ectopic pregnancy and is a life-threatening condition for both mother and child. Ectopic pregnancy can also occur if a fertilized egg implants somewhere in the uterine tube before it reaches the uterus. Basically, anywhere implantation occurs, with the exception of the normal location in the body of the uterus, is considered an ectopic pregnancy.
The connection between the surface of the ovary and the uterine tube is not fully enclosed. At ovulation, the released oocyte must find its way into the uterine tube with the help of the fimbriae (finger-like projections from the open end of the uterine tube that move, creating a current to pull the oocyte into the uterine tube). From time to time, an oocyte does not make it into the uterine tube and ends up in the peritoneal cavity. Sperm can also find their way through the uterine tube and into the peritoneal cavity. If the egg and sperm meet and fertilization occurs, the embryo will attempt to implant wherever it can – the ovarian surface, the peritoneal surface of the uterus, the outside surface of the uterine tube, somewhere else in the abdomen. Obviously, childbirth can never occur under these circumstances. The resulting pregnancy is called an ectopic pregnancy and is a life-threatening condition for both mother and child. Ectopic pregnancy can also occur if a fertilized egg implants somewhere in the uterine tube before it reaches the uterus. Basically, anywhere implantation occurs, with the exception of the normal location in the body of the uterus, is considered an ectopic pregnancy.
Clinical Connection: Twinning
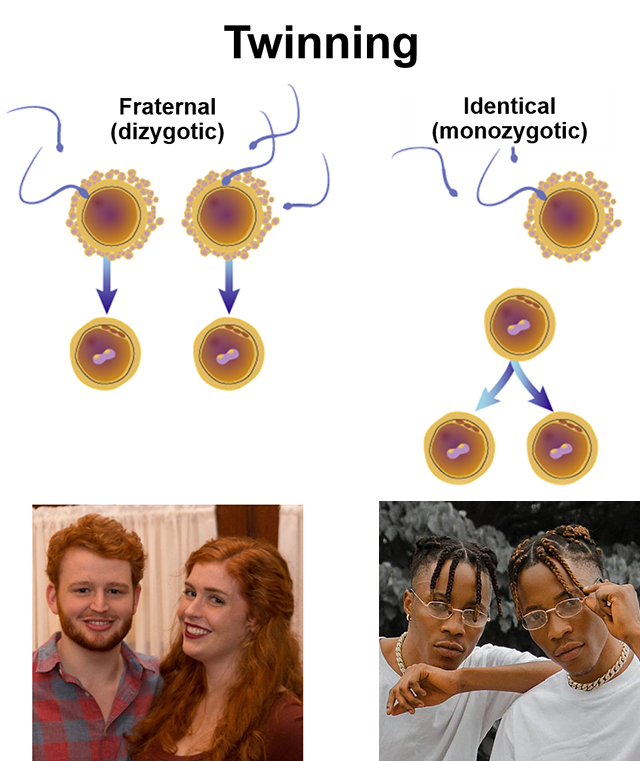 Twins occur in one of two ways. Dizygotic (fraternal) twins occur when two oocytes are released at ovulation, usually one from each ovary. Monozygotic (identical) twins occur when a single zygote splits into two embryos in an early division. Other types of multiple births follow the same pattern. For example, identical triplets result when the zygote divides, and then one of those two divides again. The vast majority of multiple births greater than two (twins) are fraternal (multiple oocytes) rather than identical.
Twins occur in one of two ways. Dizygotic (fraternal) twins occur when two oocytes are released at ovulation, usually one from each ovary. Monozygotic (identical) twins occur when a single zygote splits into two embryos in an early division. Other types of multiple births follow the same pattern. For example, identical triplets result when the zygote divides, and then one of those two divides again. The vast majority of multiple births greater than two (twins) are fraternal (multiple oocytes) rather than identical.
The Embryo – Fertilization to Gastrulation
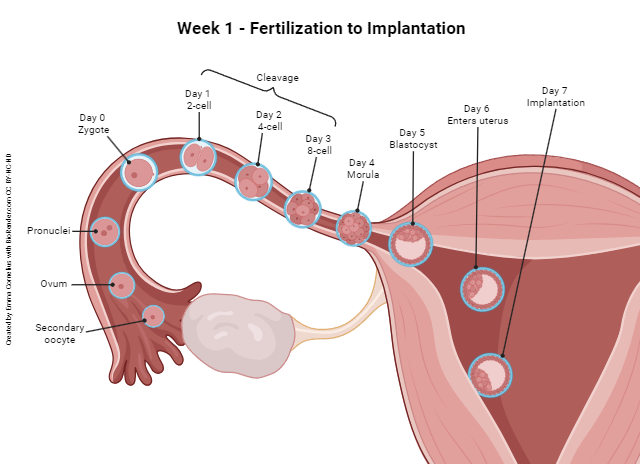
During the first week after fertilization, the embryo travels through the uterine tube into the uterus and implants. Along the way, it continues the process of mitotic cell division. (Remember that fertilization is considered day 0 for all the pregnancy-related events that will occur thereafter.)
The first division — or cleavage — of the zygote occurs on day 1 and results in two symmetrical, equal cells (two-cell embryo). It is at this point that a complete splitting of the entire embryo would result in monozygotic (identical) twins.
The next cleavage, on day 2, produces four equal, identical cells (four-cell embryo), and the next, on day 3, produces eight equal, identical cells (eight-cell embryo). This process continues with the cell number doubling a couple more times through the end of day 4, and the resulting ball of cells is called a morula (Latin: “raspberry”).
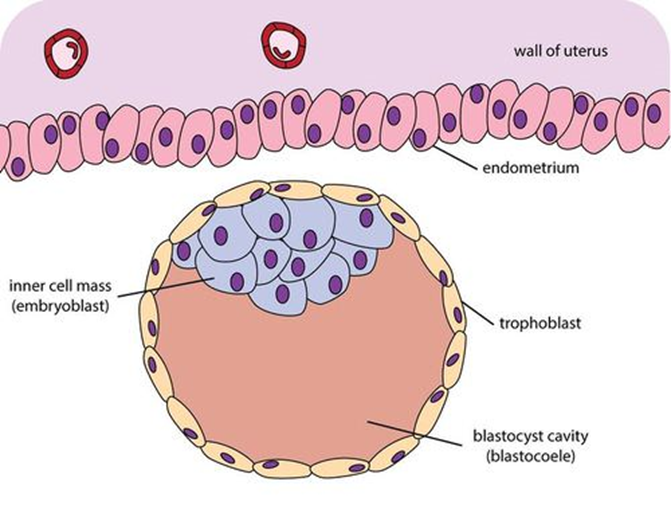 About day 5, a fluid-filled space forms within the morula. We call this now hollow ball of cells a blastocyst (Greek: blastos- “growing” + -kystis “bladder sac”) and the fluid-filled hollow space inside is the blastocele. On about day 6, the blastocyst completes its journey through the uterine tube and enters the uterus. On about day 7, the blastocyst makes contact with the endometrium (uterine lining) and begins the process of implantation.
About day 5, a fluid-filled space forms within the morula. We call this now hollow ball of cells a blastocyst (Greek: blastos- “growing” + -kystis “bladder sac”) and the fluid-filled hollow space inside is the blastocele. On about day 6, the blastocyst completes its journey through the uterine tube and enters the uterus. On about day 7, the blastocyst makes contact with the endometrium (uterine lining) and begins the process of implantation.
During all of this, the cells of the blastocyst are still dividing mitotically. When the blastocyst contacts the endometrium, chemical factors released by the endometrial tissue induce some of the blastocyst cells to congregate toward the point of contact between the blastocyst and the endometrium. This partially fills the blastocele and we refer to this collection of cells as the inner cell mass. The outer layer of cells, those that surround the inner cell mass and the “hollow” portion of the blastocyst, are now called the trophoectoderm.
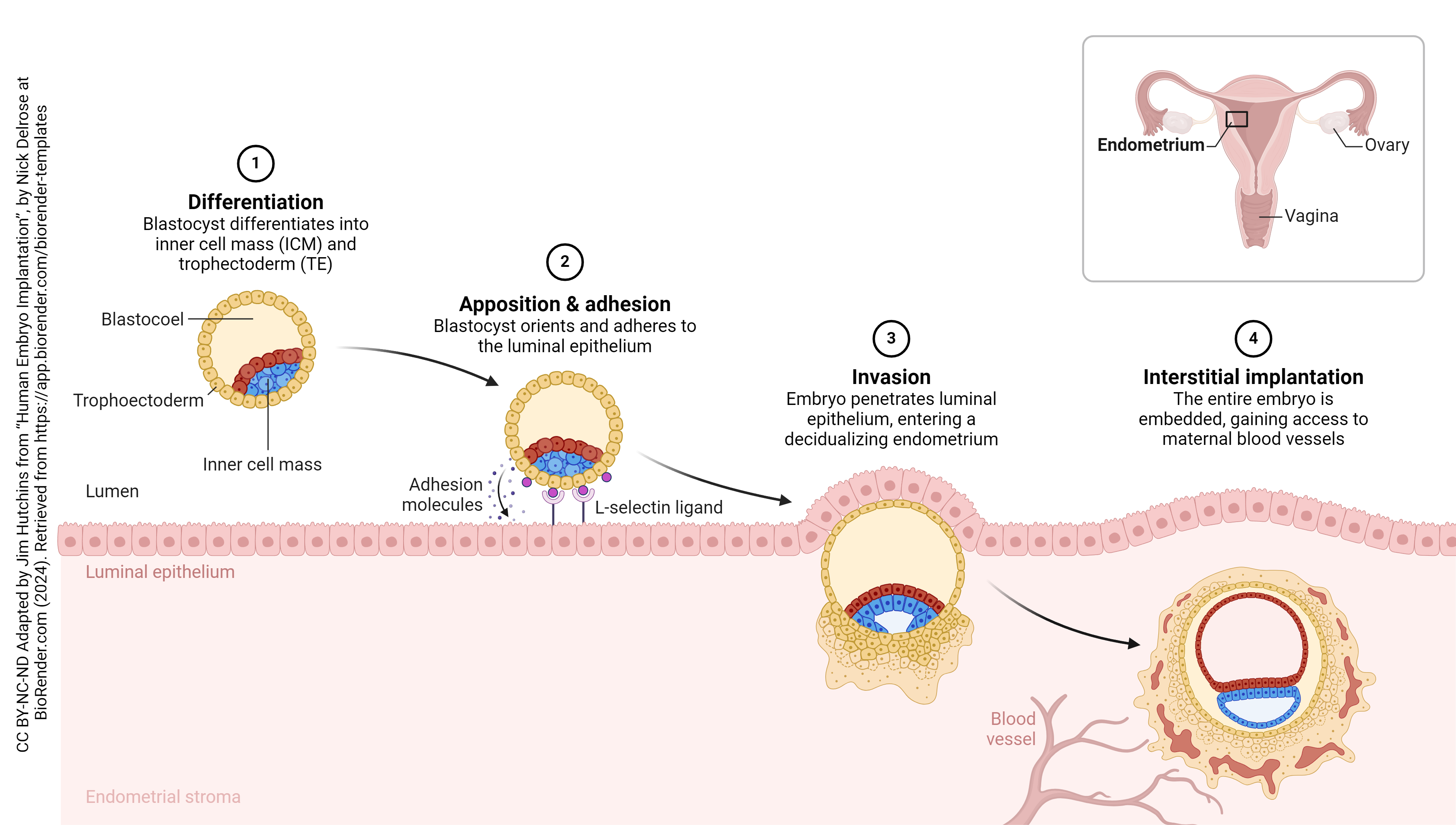
Implantation puts the embryo into direct and intimate contact with mother’s body tissues. Remember that the embryo has DNA from both mother and father and, as such, will be recognized as foreign by mom’s immune system. This presents a unique challenge – how to convince mother’s immune system not to attack, but to allow complete embryonic and fetal development without interference by inflammation, macrophages, T cells, or antibodies. We’ll find out how this accomplished in the next section, titled Extraembryonic Membranes.
Now the embryo begins its second week of development. About day 8, it is half-buried in the uterine wall and the cells of the blastocyst are arranging themselves into three layers: 1) the trophoectoderm (the outside layer of cells that surrounds the blastocele and the other two groups of cells); 2) the inner cell mass (embryonic disc); and 3) the yolk sac. Notice that yolk sac cells start out as the “surface layer” of the inner cell mass, differentiate, and then migrate up the inside surface of the trophoectoderm (white arrows on image). By day 12, this process is done and the embryo is completely buried in the endometrial tissue.
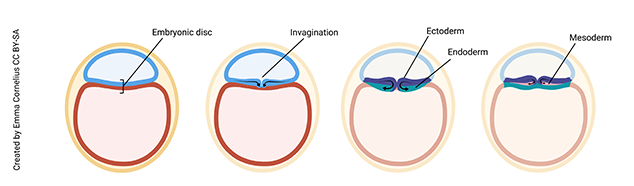
At day 16, a magical process occurs. Scientists call this gastrulation. The embryologist Lewis Wolpert, who had a bit of a flamboyant streak, famously said, “It is not birth, marriage, or death, but gastrulation which is truly the most important time in your life.”
Unfortunately, most of us don’t remember the day we gastrulated, but it turns out Wolpert was pretty much spot-on. If you don’t gastrulate, nothing further can happen. You’ll end up being resorbed by mother’s uterus and disappear without a trace. Gastrulation is the process by which the cells of the embryonic disc fold in on themselves to form three layers. From these three layers will develop all the different tissue types that make up the human body.
Note (on the image above) that the cells of the embryonic disc have moved a bit to form an invagination (groove) on the surface of the disc. Cells are migrating into the groove to form the endoderm germ layer of the embryo (yellow on image), which will develop into the digestive tract, respiratory tract, liver, and pancreas. The cells that remain (do not move into the groove) make up the ectoderm germ layer (green on image), which will become the epidermis, hair, nails, and nervous system. Finally, cells migrate into the grove to form a third layer between the ectoderm and endoderm, called the mesoderm (purple on image), which will form connective tissue, muscle, and bone.
Understanding gastrulation is important in clinical medicine. For example, if you know that skin and nerves arise from the same embryonic layer (ectoderm), it makes sense that patients with one type of nerve cell cancer (neurofibromatosis) also have defects in skin pigmentation.
Extraembryonic Membranes
Let’s go back to the time between implantation (day 6 post-conception, i.e. about 20 days after the end of the last period) and gastrulation (day 16 post-conception, or about 30 days after the end of the last period).
Day 8 post-conception
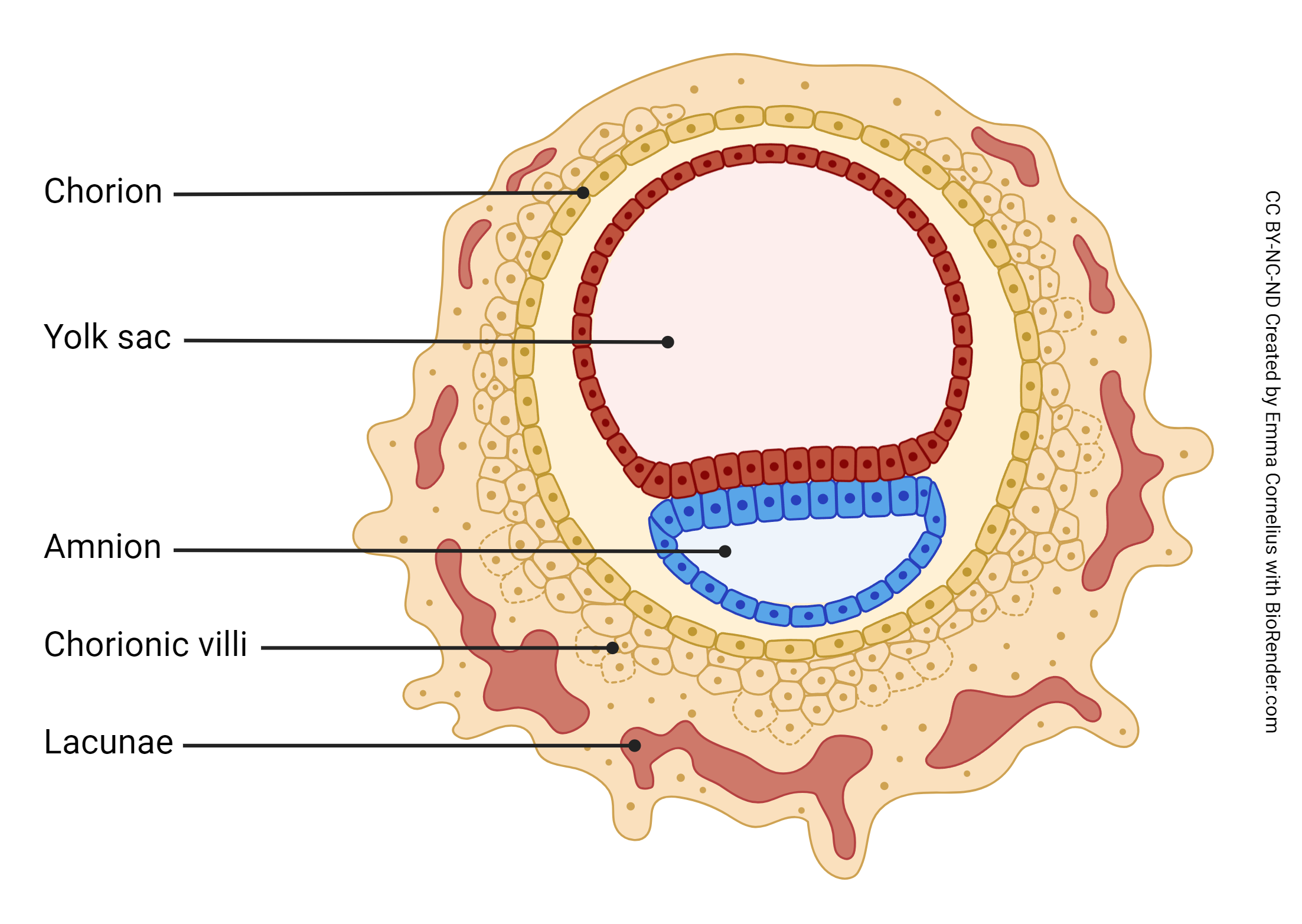
Day 8 post-conception marks the beginning of the development of the extraembryonic membranes. The trophoectoderm, the inner cell mass, and the yolk sac have developed. The extraembryonic membranes begin development at day 8 post-conception. The amniotic cavity, formed by the amniotic membrane, is blue; the yolk sac is red.
Recall the embryo’s unique challenge – to successfully convince the maternal immune system to leave it alone. This is no small task, given that the embryo must also convince maternal systems to provide nutrition and waste disposal services. Enter the extraembryonic membranes, i.e. tissues that develop from the zygote but are not part of the fetus. There are two extraembryonic membranes – the amnion and the chorion. The amnion becomes the inner layer of the amniotic sac, which completely surrounds the developing fetus, secretes amniotic fluid, and usually ruptures prior to labor onset (“I think my water just broke….”). The chorion has two roles: 1) it completely surrounds the amnion, making up the outer layer of the amniotic sac, and 2) it becomes the fetal portion of a joint project called the placenta. The placenta is the structure that solves the embryo’s unique challenge – it shields the embryo from mom’s immune system and provides the means for exchange of oxygen, nutrients, and waste.
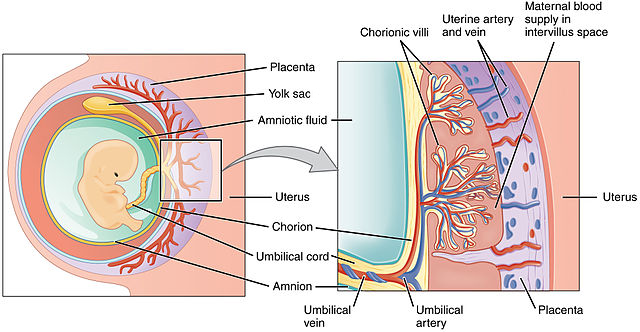
The placenta is half embryonic tissue and half maternal tissue. Placenta formation (placentation) begins when the trophoectoderm of the blastocyst contacts, fuses with, and then invades the endometrial epithelium and underlying endometrial tissue (implantation). Numerous lacunae form in the endometrium surrounding the implantation site as the endometrium transforms into a specialized tissue called decidua. The trophoectoderm gives rise to the chorion and loops of blood vessels called chorionic villi (singular: chorionic villus). The chorionic villi push into the endometrium and the lacunae; this becomes the interface between maternal and fetal circulation, with the maternal blood in the lacunae and the fetal blood flowing through the chorionic villi.
The placenta becomes a 500 g structure by the time of birth. In addition to oxygen, nutrient, and waste exchange, the placenta also allows the transfer of maternal immunoglobulins (IgG only) to the developing fetus via facilitated diffusion. These will make up the newborn’s natural passive immunity. Along with secretory IgA from breast milk, these help prevent infections in the immunologically naïve baby pending development of its own active immunity, whether spurred naturally (infections) or artificially (immunizations).
Blood flow through the placenta is as follows:
- Maternal side – maternal circulatory system to uterine artery to intervillus space to uterine vein to maternal circulatory system
- Fetal side – fetus to umbilical artery to chorionic villi to umbilical vein to fetus
Note that the oxygenated blood (represented by a red vessel) flows from placenta to child through the umbilical vein in the umbilical cord. Is this a labeling error? Since it is oxygenated blood, should it be labeled umbilical artery? Or are there any other examples of oxygenated blood in a vein, or deoxygenated blood in an artery?
Oxygen and nutrients move down their concentration gradient from mom’s blood in the intervillus space, across a single layer of endothelium, into baby’s blood in the chorionic villi. Wastes from baby also move down their concentration gradients but in the opposite direction, from baby’s blood in chorionic villi to mom’s blood in the intervillus space.
Clinical Connection: Chorionic Villus Sampling
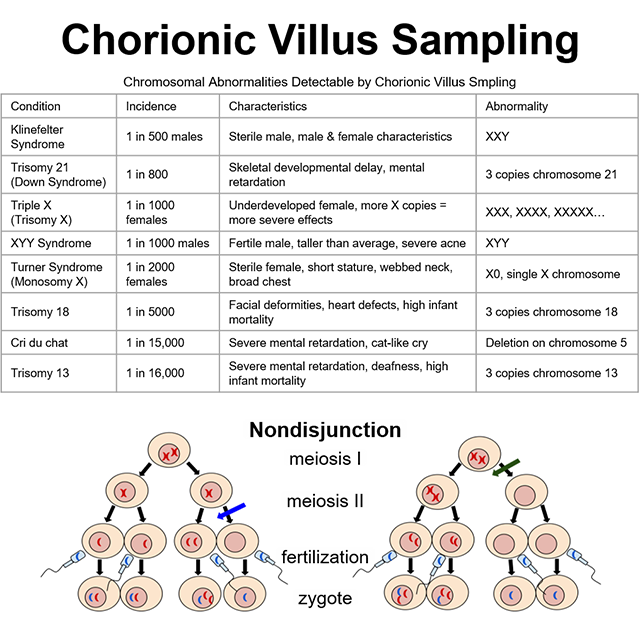
The chorionic villi are important as the first structures of the developing embryo that can be biopsied to detect genetic defects that may be present. Since the chorionic villi are an extraembryonic tissue, any chromosomal abnormalities found in chorionic villi cells will also be present in fetal cells. Chorionic villi sampling can detect many different chromosomal abnormalities, as well as other genetic conditions. A chorionic villi biopsy is taken and the karyotype is assessed. Extra or missing chromosomes are thus easily observed.
One of the most common chromosomal abnormalities detected by chorionic villus sampling is Trisomy 21, or Down Syndrome. Trisomy 21 and the other chromosomal abnormalities listed in the accompanying table result from nondisjunction (chromatid separation failure) during meiosis. Nondisjunction may occur during metaphase I or metaphase II, as shown in the image. In either case, the resulting gamete either gains or loses one copy of one chromosome.
If that gamete joins with the opposite gender’s gamete to form a zygote, the zygote will have either three copies or only one copy of that particular chromosome. Three copies is called Trisomy and one copy is called Monosomy, in both cases followed by the affected chromosome number (eg, Trisomy 21 means three copies of chromosome 21).
In addition to somatic chromosomes (chromosomes 1-22), nondisjunction can also occur with sex chromosomes (X and Y). For example, nondisjunction of the X chromosome in female gametogenesis would produce a gamete with either XX or O (nothing). The zygote formed by an XX gamete would be either XXX or XXY, depending on whether the male gamete contributed an X or a Y. Likewise, the zygote formed by an O gamete would be either XO or YO, again, depending on whether the male gamete contributed an X or a Y. You can find the conditions of XXX, XXY, and XO listed on the table; YO is incompatible with life and is, therefore, not included in the table.
Organogenesis & Fetal Growth
The embryonic period is defined as fertilization through Week 8. This time period is also the critical period for the development of many organs, such as the heart and brain, because the embryo is extremely susceptible to external influences, such as toxins or disease, which can profoundly alter development. Within this critical period, the organs that make up the embryo are especially at risk of permanent damage from external exposures during organogenesis.
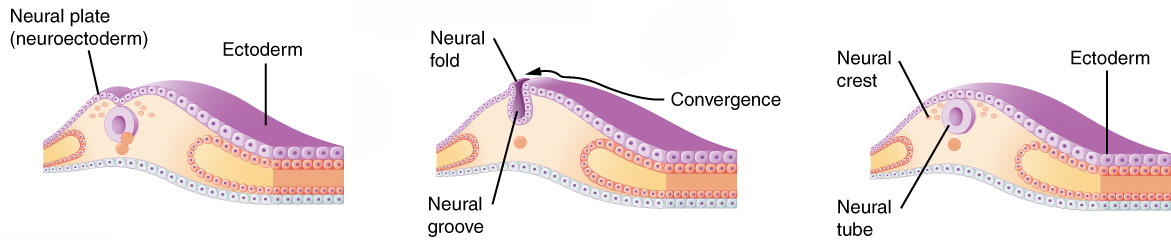
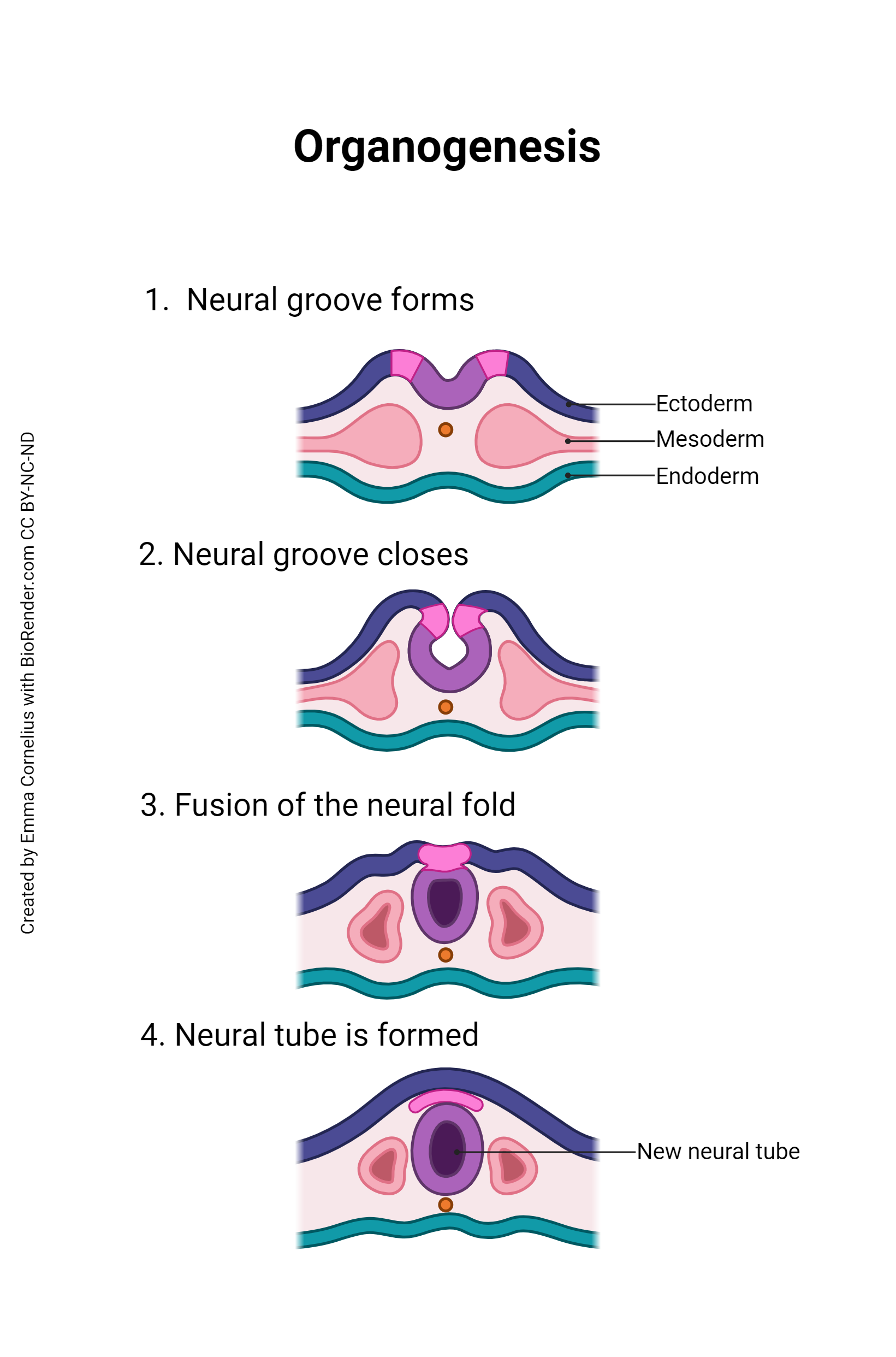
Once gastrulation is complete and the three embryonic germ layers are formed (after Day 16), organogenesis can begin. The individual organs which will make up the human body start to develop from precursor tissues found in the appropriate germ layer. For example, we’ve already said the nervous system derives from the ectoderm. Nervous system organogenesis begins with the appearance of the neural groove, a prominent feature of the dorsal surface of the embryo. The lips of this groove rise and meet to form the neural tube. The neural tube will twist and fold to form the brain and spinal cord.
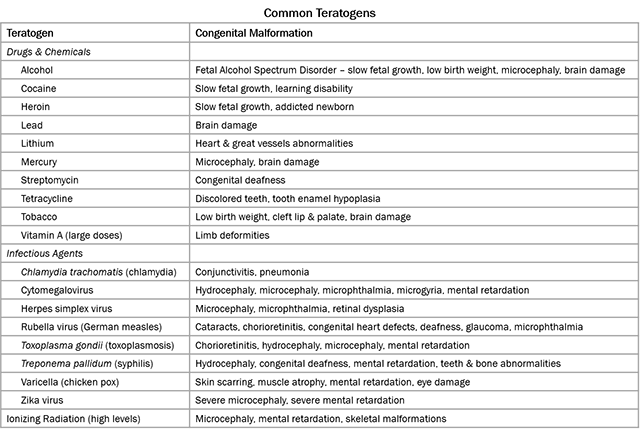
During organogenesis, even small errors can result in huge problems later on. For example, the developing brain must have 100 billion neurons at birth; to meet that need, during Week 3, the developing brain is growing at a rate of 250,000 new nerve cells per minute. Exposure to harmful substances during such rapid development can cause developmental errors. The pathogens and chemical agents that disturb these early developmental processes, cause errors, and result in birth defects, are called teratogens (Greek teras- “monster” + -genesis “creation”).
The most common teratogen, world-wide, is alcohol. Consumption of alcohol by a pregnant woman can result in the birth defect Fetal Alcohol Spectrum Disorder (FASD), which has an estimated global prevalence of about 7.7 per 1000 population. Contrary to popular belief, there is no safe amount of alcohol during pregnancy and there is no time during pregnancy when it is safe to consume alcohol. Other examples of teratogens include rubella (virus, causes rubella, aka German measles), cytomegalovirus (virus, usually asymptomatic, teratogenic if mother infected during early pregnancy), Zika (virus, mosquito-borne), Toxoplasma (parasite, causes toxoplasmosis, common in cat feces), radiation, and other chemicals.
The fetal period, defined as Week 9 through birth (which usually occurs during Week 40), is characterized primarily by rapid growth. Development does continue, but it is the continuation of a developmental plan initiated during the embryonic period. If teratogen exposure causes changes to the plan during organogenesis, those changes permanently alter the developmental plan for that tissue, organ, or system and fetal growth will continue down that altered path toward the associated birth defect.e down that altered path toward the associated birth defect.

