Respiratory pH and Control of Blood Gases
Objective 10
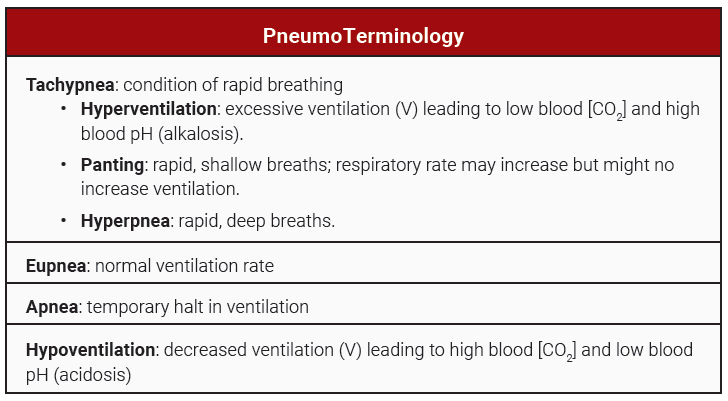
Define hyperventilation, hypoventilation, panting, eupnea, hyperpnea and apnea. Explain homeostatic mechanisms involved in control of blood gases and pH.
 Now that we have a foundation of buffers and the control centers for respiration, we are ready to discuss respiratory regulation of blood gases and pH.
Now that we have a foundation of buffers and the control centers for respiration, we are ready to discuss respiratory regulation of blood gases and pH.
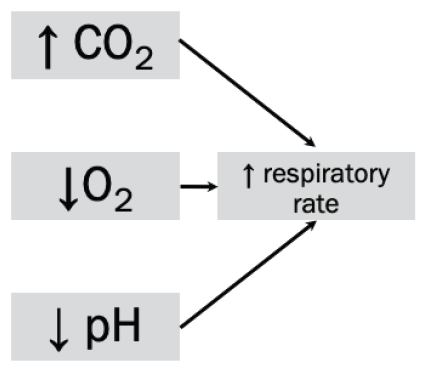
The body has a built-in mechanism to regulate CO2 , O2 , and H+ levels in the blood. Chemoreceptors send input to the inspiratory center to increase respirations if CO2 and/or H+ are high or PO2 is low.
Increased CO2, increased H+ (i.e. lower pH), or decreased O2 would lead to an increase in rate and depth of breathing.
Can you die by holding your breath?
If the PO2 drops below a normal level of 100 mm Hg, chemoreceptors are stimulated. You are consciously holding your breath through the influence of the motor cortex. If your PO2 drops below a certain level, fainting (syncope) follows, and the brainstem will take over the work of breathing while you are unconscious.
A low PCO2 (below 40 mmHg) does not signal chemoreceptors and will not stimulate the inspiratory center. (The inspiratory center responds to high CO2 , not low CO2 ). So, if you hyperventilate, blowing off CO2 and increasing O2 the inspiratory center slows and you can hold your breath longer.
Swimmers were once encouraged to hyperventilate before swimming to be able to hold their breath longer.
Why is it dangerous to hyperventilate and then swim underwater?
This is dangerous because O2 levels may fall to a dangerously low condition before the chemoreceptor reflex is activated, causing fainting (syncope). Passing out under water is not a good idea as drowning may occur!
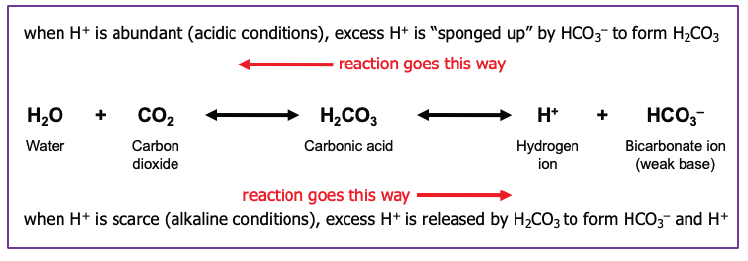
Because CO2 easily converts to an acid, the respiratory system can help control blood pH by either speeding up breathing to get rid of CO2 or slowing down breathing to retain CO2.
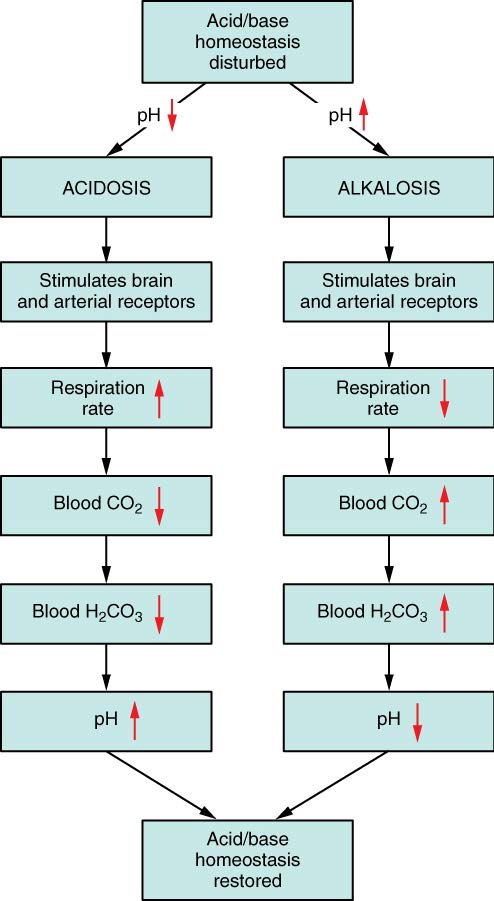
A condition of too much alkali (too high a pH, too low a [H+]) in the blood is called alkalosis.
Hyperventilation, or excessive ventilation is a mechanism used by the body to lower the blood [H+]. Hyperventilation during your favorite exam may also lead to alkalosis. (You knew exams were bad for your health!)
A condition of too much acid (too low a pH, too high a [H+]) in the blood is called acidosis.
Hypoventilation retains more CO2. While the body may use this mechanism in conditions of alkalosis to decrease the pH, if it lasts more than a few seconds it usually results from pathology. Patients with chronic obstructive pulmonary diseases such as emphysema have difficulty exhaling and often build up CO2 leading to acidosis.
Media Attributions
- U17-059 Reflex Gases © Hutchins, Jim is licensed under a CC BY-SA (Attribution ShareAlike) license
- U17-057 Bicarbonate Carbonic Acid © Hutchins, Jim is licensed under a CC BY-SA (Attribution ShareAlike) license
- U17-062 Response Respiratory System to Acidosis or Alkalosis © Betts, J. Gordon; Young, Kelly A.; Wise, James A.; Johnson, Eddie; Poe, Brandon; Kruse, Dean H. Korol, Oksana; Johnson, Jody E.; Womble, Mark & DeSaix, Peter is licensed under a CC BY (Attribution) license
- U17-063 Pneumoterminology Table © Burr, Justin is licensed under a CC BY-SA (Attribution ShareAlike) license

