Required Reading for Week 1 – HDFS 1500 Spring 2025
Chromosomal Abnormalities and Genetic Testing
Chromosomal Abnormalities
A chromosomal abnormality occurs when a child inherits too many or too few chromosomes. The most common cause of chromosomal abnormalities is the age of the mother. A 20-year-old woman has a 1 in 800 chance of having a child with a common chromosomal abnormality. A woman of 44, however, has a one in 16 chance. It is believed that the problem occurs when the ovum is ripening prior to ovulation each month. As the mother ages, the ovum is more likely to suffer abnormalities at this time.
Another common cause of chromosomal abnormalities occurs because the gametes do not divide evenly when they are forming. Therefore, some cells have more than 46 chromosomes. In fact, it is believed that close to half of all zygotes have an odd number of chromosomes. Most of these zygotes fail to develop and are spontaneously aborted by the body. If the abnormal number occurs on pair # 21 or # 23, however, the individual may have certain physical or other abnormalities.
An altered chromosome structure may take several different forms, and result in various disorders or malignancies:
-
Deletions: A portion of the chromosome is missing or deleted. Known disorders in humans include Wolf-Hirschhorn syndrome, which is caused by partial deletion of the short arm of chromosome 4; and Jacobsen syndrome, also called the terminal 11q deletion disorder.
- Duplications: A portion of the chromosome is duplicated, resulting in extra genetic material. Known human disorders include Charcot-Marie-Tooth disease type 1A, which may be caused by duplication of the gene encoding peripheral myelin protein 22 (PMP22) on chromosome 17.

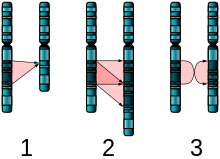
Figure 6. The two major two-chromosome mutations: insertion (1) and Translocation (2). - Translocations: A portion of one chromosome is transferred to another chromosome. There are two main types of translocations:
- Reciprocal translocation: Segments from two different chromosomes have been exchanged.
- Robertsonian translocation: An entire chromosome has attached to another at the centromere – in humans, these only occur with chromosomes 13, 14, 15, 21, and 22.
- Inversions: A portion of the chromosome has broken off, turned upside down, and reattached, therefore the genetic material is inverted.
- Insertions: A portion of one chromosome has been deleted from its normal place and inserted into another chromosome.
- Rings: A portion of a chromosome has broken off and formed a circle or ring. This can happen with or without loss of genetic material.
- Isochromosome: Formed by the mirror image copy of a chromosome segment including the centromere.
One of the most common chromosomal abnormalities is on pair # 21. Trisomy 21 occurs when there are three rather than two chromosomes on #21. A person with Down syndrome has distinct facial features, intellectual disability, and oftentimes heart and gastrointestinal disorders. Symptoms vary from person to person and can range from mild to severe. With early intervention, the life expectancy of persons with Down syndrome has increased in recent years. Keep in mind that there is as much variation in people with Down Syndrome as in most populations and those differences need to be recognized and appreciated.
When the chromosomal abnormality is on pair #23, the result is a sex-linked chromosomal abnormality. A person might have XXY, XYY, XXX, XO, or 45 or 47 chromosomes as a result. Two of the more common sex-linked chromosomal disorders are Turner syndrome and Klinefelter syndrome. Turner’s syndrome occurs in 1 of every 2,500 live female births (Carroll, 2007) when an ovum which lacks a chromosome is fertilized by a sperm with an X chromosome. The resulting zygote has an XO composition. Fertilization by a Y sperm is not viable. Turner syndrome affects cognitive functioning and sexual maturation. The external genitalia appear normal, but breasts and ovaries do not develop fully and the woman does not menstruate. Turner’s syndrome also results in short stature and other physical characteristics. Klinefelter syndrome (XXY) occurs in 1 out of 700 live male births and results when an ovum containing an extra X chromosome is fertilized by a Y sperm. The Y chromosome stimulates the growth of male genitalia, but the additional X chromosome inhibits this development. An individual with Klinefelter syndrome has some breast development, infertility (this is the most common cause of infertility in males), and has low levels of testosterone.
Prenatal Testing
Prenatal testing consists of prenatal screening and prenatal diagnosis, which are aspects of prenatal care that focus on detecting problems with the pregnancy as early as possible. These may be anatomic and physiologic problems with the health of the zygote, embryo, or fetus, either before gestation even starts or as early in gestation as practical. Prenatal screening focuses on finding problems among a large population with affordable and noninvasive methods. The most common screening procedures are routine ultrasounds, blood tests, and blood pressure measurement. Prenatal diagnosis focuses on pursuing additional detailed information once a particular problem has been found, and can sometimes be more invasive.
Screening can detect problems such as neural tube defects, anatomical defects, chromosome abnormalities, and gene mutations that would lead to genetic disorders and birth defects, such as spina bifida, cleft palate, Downs Syndrome, Tay–Sachs disease, sickle cell anemia, thalassemia, cystic fibrosis, muscular dystrophy, and fragile X syndrome. Some tests are designed to discover problems which primarily affect the health of the mother, such as PAPP-A to detect pre-eclampsia or glucose tolerance tests to diagnose gestational diabetes. Screening can also detect anatomical defects such as hydrocephalus, anencephaly, heart defects, and amniotic band syndrome.
Common prenatal diagnosis procedures include amniocentesis and chorionic villus sampling. Because of the miscarriage and fetal damage risks associated with amniocentesis and CVS procedures, many women prefer to first undergo screening so they can find out if the fetus’ risk of birth defects is high enough to justify the risks of invasive testing. Screening tests yield a risk score which represents the chance that the baby has the birth defect; the most common threshold for high-risk is 1:270. A risk score of 1:300 would, therefore, be considered low-risk by many physicians. However, the trade-off between the risk of birth defects and risk of complications from invasive testing is relative and subjective; some parents may decide that even a 1:1000 risk of birth defects warrants an invasive test while others wouldn’t opt for an invasive test even if they had a 1:10 risk score.
There are three main purposes of prenatal diagnosis: (1) to enable timely medical or surgical treatment of a condition before or after birth, (2) to give the parents the chance to abort a fetus with the diagnosed condition, and (3) to give parents the chance to prepare psychologically, socially, financially, and medically for a baby with a health problem or disability, or for the likelihood of a stillbirth. Having this information in advance of birth means that healthcare staff, as well as parents, can better prepare themselves for the delivery of a child with a health problem. For example, Down Syndrome is associated with cardiac defects that may need intervention immediately upon birth.
The American College of Obstetricians and Gynecologists (ACOG) guidelines currently recommend that all pregnant women, regardless of age, be offered invasive testing to obtain a definitive diagnosis of certain birth defects. Therefore, most physicians offer diagnostic testing to all their patients, with or without prior screening and let the patient decide.
watch it
Watch this video to learn more about prenatal testing and screening during pregnancy.
Behavioral Genetics
Behavioral Genetics
Behavioral geneticists study how individual differences arise, in the present, through the interaction of genes and the environment. When studying human behavior, behavioral geneticists often employ twin and adoption studies to research questions of interest. Twin studies compare the rates that a given behavioral trait is shared among identical and fraternal twins; adoption studies compare those rates among biologically related relatives and adopted relatives. Both approaches provide some insight into the relative importance of genes and environment for the expression of a given trait.
Nature or Nurture?
For decades, scholars have carried on the “nature/nurture” debate. For any particular feature, those on the “nature” side would argue that heredity plays the most important role in bringing about that feature. Those on the “nurture” side would argue that one’s environment is most significant in shaping the way we are. This debate continues in questions about what makes us masculine or feminine (Lippa, 2002), concerns about vision (Mutti, Kadnik, & Adams, 1996), and many other developmental issues.
Most scholars agree that there is a constant interplay between the two forces. It is difficult to isolate the root of any single behavior as a result solely of nature or nurture, and most scholars believe that even determining the extent to which nature or nurture impacts a human feature is difficult to answer. In fact, almost all human features are polygenic (a result of many genes) and multifactorial (a result of many factors, both genetic and environmental). It is as if one’s genetic make-up sets up a range of possibilities, which may or may not be realized depending upon one’s environmental experiences. For instance, a person might be genetically predisposed to develop diabetes, but the person’s lifestyle may help bring about the disease.
When you think about your own family history, it is easy to see that there are certain personality traits, behavioral characteristics, and medical conditions that are more common than others. This is the reason that doctors ask you about your family medical history. While genetic predisposition is important to consider, there are some family members who, for a variety of reasons, seemed to defy the odds of developing these conditions. These differences can be explained in part by the effect of epigenetic (above the genome) changes.
The Epigenetic Framework
The term “epigenetic” has been used in developmental psychology to describe psychological development as the result of an ongoing, bi-directional interchange between heredity and the environment. Gottlieb (1998; 2000; 2002) suggests an analytic framework for the nature/nurture debate that recognizes the interplay between the environment, behavior, and genetic expression. This bidirectional interplay suggests that the environment can effect the expression of genes just as genetic predispositions can impact a person’s potentials. Likewise, environmental circumstances can trigger symptoms of a genetic disorder. For example, a person predisposed genetically for type 2 diabetes may trigger the disease through poor diet and little exercise.
The developmental psychologist Erik Erikson wrote of an epigenetic principle in his book Identity: Youth and Crisis (1968), encompassing the notion that we develop through an unfolding of our personality in predetermined stages, and that our environment and surrounding culture influence how we progress through these stages. This biological unfolding in relation to our socio-cultural settings is done in stages of psychosocial development, where “progress through each stage is in part determined by our success, or lack of success, in all the previous stages.”
In typical human families, children’s biological parents raise them, so it is very difficult to know whether children act like their parents due to genetic (nature) or environmental (nurture) reasons. Nevertheless, despite our restrictions on setting up human-based experiments, we do see real-world examples of nature-nurture at work in the human sphere—though they only provide partial answers to our many questions. The science of how genes and environments work together to influence behavior is called behavioral genetics. The easiest opportunity we have to observe this is the adoption study. When children are put up for adoption, the parents who give birth to them are no longer the parents who raise them. Children aren’t assigned to random adoptive parents in order to suit the particular interests of a scientist but adoption still tells us some interesting things, or at least confirms some basic expectations. For instance, if the biological child of tall parents were adopted into a family of short people, do you suppose the child’s growth would be affected? What about the biological child of a Spanish-speaking family adopted at birth into an English-speaking family? What language would you expect the child to speak? And what might these outcomes tell you about the difference between height and language in terms of nature-nurture?
Monozygotic and Dizygotic Twins
Another option for observing nature-nurture in humans involves twin studies. To analyze nature–nurture using twins, we compare the similarity of monozygotic and dizygotic pairs. Monozygotic twins occur when a single zygote or fertilized egg splits apart in the first two weeks of development. The result is the creation of two separate but genetically identical offspring. About one-third of twins are monozygotic twins. Monozygotic twins occur in birthing at a rate of about 3 in every 1000 deliveries worldwide (about 0.3% of the world population). Monozygotic twins are genetically nearly identical and they are always the same sex unless there has been a mutation during development. The children of monozygotic twins test genetically as half-siblings (or full siblings, if a pair of monozygotic twins reproduces with another pair of identical twins or with the same person), rather than first cousins.
Sometimes two eggs or ova are released and fertilized by two separate sperm. The result is dizygotic or fraternal twins. About two-thirds of twins are dizygotic. These two individuals share the same amount of genetic material as would any two children from the same mother and father. Older mothers are more likely to have dizygotic twins than are younger mothers and couples who use fertility drugs are also more likely to give birth to dizygotic twins. Consequently, there has been an increase in the number of fraternal twins in recent years (Bortolus et al., 1999). In vitro fertilization (IVF) techniques are more likely to create dizygotic twins. For IVF deliveries, there are nearly 21 pairs of twins for every 1,000.
In the uterus, a majority of monozygotic twins (60–70%) share the same placenta but have separate amniotic sacs. The placenta is a temporary organ that connects the developing fetus via the umbilical cord to the uterine wall to allow nutrient uptake, thermo-regulation, waste elimination, and gas exchange via the mother’s blood supply. The amniotic sac (also called the bag of waters or the membranes), is a thin but tough transparent pair of membranes that hold a developing embryo (and later fetus) until shortly before birth. In 18–30% of monozygotic twins each fetus has a separate placenta and a separate amniotic sac. A small number (1–2%) of monozygotic twins share the same placenta and amniotic sac. Fraternal twins each have their own placenta and own amniotic sac.

Monozygotic (one egg/identical) twins can be categorized into four types depending on the timing of the separation and duplication of cells. Various types of chorionicity and amniosity (how the baby’s sac looks) in monozygotic twins are a result of when the fertilized egg divides. This is known as placentation.
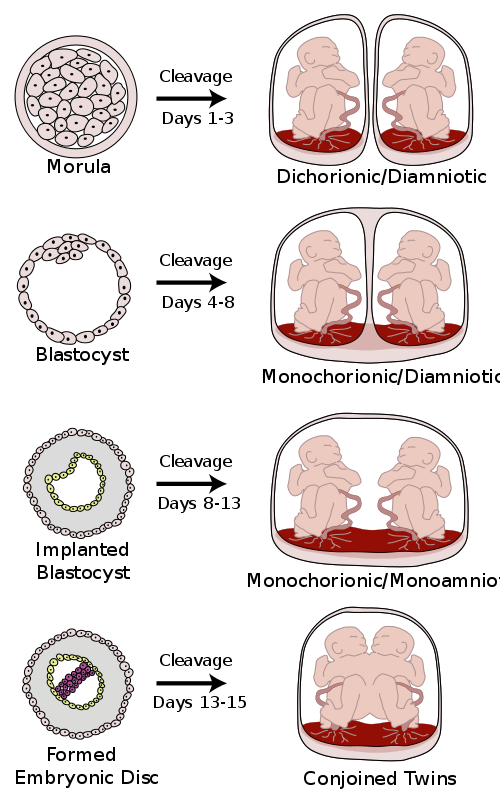
Conjoined twins
Conjoined twins are monozygotic twins whose bodies are joined together during pregnancy. This occurs when the zygote starts to split after day 12 following fertilization and fails to separate completely. This condition occurs in about 1 in 50,000 human pregnancies. Most conjoined twins are now evaluated for surgery to attempt to separate them into separate functional bodies. The degree of difficulty rises if a vital organ or structure is shared between twins, such as the brain, heart or liver.
Vanishing twins
Researchers suspect that as many as 1 in 8 pregnancies start out as multiples, but only a single fetus is brought to full term because the other fetus has died very early in the pregnancy and has not been detected or recorded. Early obstetric ultrasonography exams sometimes reveal an “extra” fetus, which fails to develop and instead disintegrates and vanishes in the uterus. There are several reasons for the “vanishing” fetus, including it being embodied or absorbed by the other fetus, placenta or the mother. This is known as vanishing twin syndrome. Also, in an unknown proportion of cases, two zygotes may fuse soon after fertilization, resulting in a single chimeric embryo, and, later, fetus.
Twin Studies
Using the features of height and spoken language as examples, let’s take a look at how nature and nurture apply: identical twins, unsurprisingly, are almost perfectly similar for height. The heights of fraternal twins, however, are like any other sibling pairs: more similar to each other than to people from other families, but hardly identical. This contrast between twin types gives us a clue about the role genetics plays in determining height.

Now consider spoken language. If one identical twin speaks Spanish at home, the co-twin with whom she is raised almost certainly does too. But the same would be true for a pair of fraternal twins raised together. In terms of spoken language, fraternal twins are just as similar as identical twins, so it appears that the genetic match of identical twins doesn’t make much difference.
Twin and adoption studies are two instances of a much broader class of methods for observing nature-nurture called quantitative genetics, the scientific discipline in which similarities among individuals are analyzed based on how biologically related they are. We can do these studies with siblings and half-siblings, cousins, and twins who have been separated at birth and raised separately (Bouchard, Lykken, McGue, & Segal, 1990). Such twins are very rare and play a smaller role than is commonly believed in the science of nature–nurture, or with entire extended families (Plomin, DeFries, Knopik, & Neiderhiser, 2012).
It would be satisfying to be able to say that nature–nurture studies have given us conclusive and complete evidence about where traits come from, with some traits clearly resulting from genetics and others almost entirely from environmental factors, such as child-rearing practices and personal will; but that is not the case. Instead, everything has turned out to have some footing in genetics. The more genetically-related people are, the more similar they are—for everything: height, weight, intelligence, personality, mental illness, etc. Sure, it seems like common sense that some traits have a genetic bias. For example, adopted children resemble their biological parents even if they have never met them, and identical twins are more similar to each other than are fraternal twins. And while certain psychological traits, such as personality or mental illness (e.g., schizophrenia), seem reasonably influenced by genetics, it turns out that the same is true for political attitudes, how much television people watch (Plomin, Corley, DeFries, & Fulker, 1990), and whether or not they get divorced (McGue & Lykken, 1992).
Environmental Risks
Teratology
Good prenatal care is essential. The developing child is most at risk for some of the most severe problems during the first three months of development. Unfortunately, this is a time at which most mothers are unaware that they are pregnant. It is estimated that 10% of all birth defects are caused by a prenatal exposure or teratogen. Teratogens are factors that can contribute to birth defects which include some maternal diseases, drugs, alcohol, and stress. These exposures can also include environmental and occupational exposures. Today, we know many of the factors that can jeopardize the health of the developing child. Teratogen-caused birth defects are potentially preventable.
The study of factors that contribute to birth defects is called teratology. Teratogens are usually discovered after an increased prevalence of a particular birth defect. For example, in the early 1960’s, a drug known as thalidomide was used to treat morning sickness. Exposure of the fetus during this early stage of development resulted in cases of phocomelia, a congenital malformation in which the hands and feet are attached to abbreviated arms and legs.
A Look at Some Teratogens
Alcohol

One of the most commonly used teratogens is alcohol. Because half of all pregnancies in the United States are unplanned, it is recommended that women of child-bearing age take great caution against drinking alcohol when not using birth control and when pregnant (Surgeon General’s Advisory on Alcohol Use During Pregnancy, 2005). Alcohol consumption, particularly during the second month of prenatal development, but at any point during pregnancy, may lead to neurocognitive and behavioral difficulties that can last a lifetime.
There is no acceptable safe limit for alcohol use during pregnancy, but binge drinking (5 or more drinks on a single occasion) or having 7 or more drinks during a single week places a child at particularly high risk. In extreme cases, alcohol consumption can lead to fetal death, but more frequently it can result in fetal alcohol spectrum disorders (FASD). This terminology is now used when looking at the effects of exposure and replaces the term fetal alcohol syndrome. It is preferred because it recognizes that symptoms occur on a spectrum and that all individuals do not have the same characteristics. Children with FASD share certain physical features such as flattened noses, small eye openings, small heads, intellectual developmental delays, and behavioral problems. Those with FASD are more at risk for lifelong problems such as criminal behavior, psychiatric problems, and unemployment (CDC, 2006).
The terms alcohol-related neurological disorder (ARND) and alcohol-related birth defects (ARBD) have replaced the term Fetal Alcohol Effects to refer to those with less extreme symptoms of FASD. ARBD include kidney, bone and heart problems.
Tobacco
Smoking is also considered a teratogen because nicotine travels through the placenta to the fetus. When the mother smokes, the developing baby experiences a reduction in blood oxygen levels. Tobacco use during pregnancy has been associated with low birth weight, placenta previa, birth defects, preterm delivery, fetal growth restriction, and sudden infant death syndrome. Smoking in the month before getting pregnant and throughout pregnancy increases the chances of these risks. Quitting smoking before getting pregnant is best. However, for women who are already pregnant, quitting as early as possible can still help protect against some health problems for the mother and baby.[1]
Drugs
Prescription, over-the-counter, or recreational drugs can have serious teratogenic effects. In general, if medication is required, the lowest dose possible should be used. Combination drug therapies and first trimester exposures should be avoided. Almost three percent of pregnant women use illicit drugs such as marijuana, cocaine, Ecstasy and other amphetamines, and heroin. These drugs can cause low birth-weight, withdrawal symptoms, birth defects, or learning or behavioral problems. Babies born with a heroin addiction need heroin just like an adult addict. The child will need to be gradually weaned from the heroin under medical supervision; otherwise, the child could have seizures and die.
Environmental Chemicals
Environmental chemicals can include an exposure to a wide array of agents including pollution, organic mercury compounds, herbicides, and industrial solvents. Some environmental pollutants of major concern include lead poisoning, which is connected with low birth weight and slowed neurological development. Children who live in older housing in which lead-based paints have been used have been known to eat peeling paint chips thus being exposed to lead. The chemicals in certain herbicides are also potentially damaging. Radiation is another environmental hazard that a pregnant woman must be aware of. If a mother is exposed to radiation, particularly during the first three months of pregnancy, the child may suffer some congenital deformities. There is also an increased risk of miscarriage and stillbirth. Mercury leads to physical deformities and intellectual disabilities (Dietrich, 1999).
Sexually Transmitted Infections
Sexually transmitted infections (STIs) can complicate pregnancy and may have serious effects on both the mother and the developing baby. Most prenatal care today includes testing for STIs, and early detection is important. STIs, such as chlamydia, gonorrhea, syphilis, trichomoniasis and bacterial vaginosis can all be treated and cured with antibiotics that are safe to take during pregnancy. STIs that are caused by viruses, like genital herpes, hepatitis B, or HIV cannot be cured. However, in some cases these infections can be treated with antiviral medications or other preventive measures can be taken to reduce the risk of passing the infection to the baby.[2]
Maternal Diseases
Maternal illnesses increase the chance that a baby will be born with a birth defect or have a chronic health problem. Some of the diseases that are known to potentially have an adverse effect on the fetus include: diabetes, cytomegalovirus, toxoplasmosis, Rubella, varicella, hypothyroidism, and Strep B. If the mother contracts Rubella during the first three months of pregnancy, damage can occur in the eyes, ears, heart, or brain of the unborn child. On a positive note, Rubella has been nearly eliminated in the industrial world due to the vaccine created in 1969. Diagnosing these diseases early and receiving appropriate medical care can help improve the outcomes. Routine prenatal care now includes screening for gestational diabetes and Strep B.[3]
Maternal Stress
Stress represents the effects of any factor able to threaten the homeostasis of an organism; these either real or perceived threats are referred to as the “stressors” and comprise a long list of potentially adverse factors, which can be emotional or physical. Because of a link in blood supply between a mother and fetus, it has been found that stress can leave lasting effects on a developing fetus, even before a child is born. The best-studied outcomes of fetal exposure to maternal prenatal stress are preterm birth and low birth weight. Maternal prenatal stress is also considered responsible for a variety of changes of the child’s brain, and a risk factor for conditions such as behavioral problems, learning disorders, high levels of anxiety, attention deficit hyperactivity disorder, autism, and schizophrenia. Furthermore, maternal prenatal stress has been associated with a higher risk for a variety of immune and metabolic changes in the child such as asthma, allergic disorders, cardiovascular diseases, hypertension, hyperlipidemia, diabetes, and obesity.[4]
Factors influencing prenatal risks
There are several considerations in determining the type and amount of damage that might result from exposure to a particular teratogen (Berger, 2004). These include:
- The timing of the exposure: Structures in the body are vulnerable to the most severe damage when they are forming. If a substance is introduced during a particular structure’s critical period (time of development), the damage to that structure may be greater. For example, the ears and arms reach their critical periods at about 6 weeks after conception. If a mother exposes the embryo to certain substances during this period, the arms and ears may be malformed.
- The amount of exposure: Some substances are not harmful unless the amounts reach a certain level. The critical level depends in part on the size and metabolism of the mother.
- Genetics: Genetic make-up also plays a role on the impact a particular teratogen might have on the child. This is suggested by fraternal twin studies who are exposed to the same prenatal environment, yet do not experience the same teratogenic effects. The genetic make-up of the mother can also have an effect; some mothers may be more resistant to teratogenic effects than others.
- Being male or female: Males are more likely to experience damage due to teratogens than are females. It is believed that the Y chromosome, which contains fewer genes than the X, may have an impact.

Low Birth Weight
We have been discussing a number of teratogens associated with a low birth weight such as cocaine, tobacco, etc. A child is considered to have a low birth weight if they weigh less than 5.8 pounds (2500 grams). About 8.17 percent of babies born in the United States are of low birth weight and 1.4 percent are born very low birth weight.[5] A low birth weight baby has difficulty maintaining adequate body temperature because it lacks the fat that would otherwise provide insulation. Such a baby is also at more risk of infection. And 67 percent of these babies are also preterm which can make them more at risk for a respiratory infection. Very low birth weight babies (2 pounds or less) have an increased risk of developing cerebral palsy. Many causes of low birth weight are preventable with proper prenatal care.
Premature Birth
A child might also have a low birth weight if it is born at less than 37 weeks gestation (which qualifies it as a preterm baby). In 2016, 9.85 percent of babies born in the U.S. were preterm.[6] Early birth can be triggered by anything that disrupts the mother’s system. For instance, vaginal infections or gum disease can actually lead to premature birth because such infection causes the mother to release anti-inflammatory chemicals which, in turn, can trigger contractions. Smoking and the use of other teratogens can also lead to preterm birth.
Anoxia and Hypoxia
One of leading causes of infant brain damage is lack of oxygen shortly after birth. Hypoxia occurs when the infant is deprived of the adequate amount of oxygen, leading to mild to moderate brain damage. Apoxia occurs when the infant undergoes a total lack of oxygen, which can lead to severe brain damage. This lack of oxygen is typically caused by umbilical cord problems, birth canal problems, blocked airways, and placenta abruption. Both hypoxia and anoxia can lead to cerebral palsy and a host of other medical disorders. [7]
- Birth Defects Research and Tracking. Centers for Disease Control and Prevention. Retrieved from https://www.cdc.gov/ncbddd/birthdefects/research.html ↵
- STDs during Pregnancy - CDC Fact Sheet. Centers for Disease Control and Prevention. Retrieved from https://www.cdc.gov/std/pregnancy/stdfact-pregnancy.htm ↵
- Maternal Illness – Birth Defect Prevention for Expecting Parents. Birth Defect Research for Children. Retrieved from https://www.birthdefects.org/healthy-baby/maternal-illness/ ↵
- Douros Konstantinos, Moustaki Maria, Tsabouri Sophia, Papadopoulou Anna, Papadopoulos Marios, Priftis Kostas N. (2017). Prenatal Maternal Stress and the Risk of Asthma in Children. Frontiers in Pediatrics. Retrieved from https://www.frontiersin.org/article/10.3389/fped.2017.00202 ↵
- Birthweight and Gestation. Centers for Disease Control and Prevention (2016). Retrieved from https://www.cdc.gov/nchs/fastats/birthweight.htm ↵
- Birthweight and Gestation. Centers for Disease Control and Prevention (2016). Retrieved from https://www.cdc.gov/nchs/fastats/birthweight.htm ↵
- Benaron, Harry B.W. et al. (1960). Effect of anoxia during labor and immediately after birth on the subsequent development of the child. American Journal of Obstetrics & Gynecology, Volume 80, Issue 6, 1129 - 1142. Retrieved from https://www.ajog.org/article/0002-9378(60)90080-6/pdf ↵

